Assuming that water vapour is an ideal gas, the internal energy (ΔU) when 1 mol of water is vapourized at 1 bar pressure and 100∘C. (Molar enthalpy of vapourization of water at 1 bar and 373 K =41 kJ mol−1 and R=8.3Jmol−1K−1) will beSolution in Malayalam
4.1kJmol−1
3.7904kJmol−1
37.904kJmol−1
41.0kJmol−1
The reaction involved is H2O1→H2O(g), where Δng=1mol,ΔvapH=41kJmol−1. Now ΔU=ΔH−ΔngRT
=41kJmol−1−1×8.3×10−3kJK−1mol−1×373K=41−3.096kJmol−1=37.904kJmol−1
Topper's Solved these Questions
- CHEMICAL THERMODYNAMICS Book:BRILLIANT PUBLICATIONChapter:CHEMICAL THERMODYNAMICS Exercise:LEVEL-II
Explore 102 Videos
- CHEMICAL THERMODYNAMICS Book:BRILLIANT PUBLICATIONChapter:CHEMICAL THERMODYNAMICS Exercise:LEVEL-II (Assertion-Reason Type)
Explore 18 Videos
- CHEMICAL THERMODYNAMICS Book:BRILLIANT PUBLICATIONChapter:CHEMICAL THERMODYNAMICS Exercise:LEVEL-III
Explore 51 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:BRILLIANT PUBLICATIONChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:QUESTION (LEVEL -ll)
Explore 42 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:BRILLIANT PUBLICATIONChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:QUESTIONS (LEVEL-III ( Linked Comprehension Type ))
Explore 12 Videos
Similar Questions
Which of the following statements is correct?
View SolutionAssertion : Heat of neutralisation of HNO3, and NaOH is almost same as that of HCl and KOH.
Reason : Both HNO3, and HCl are strong acids and NaOH and KOH are strong bases. : if both (A) and (R) are correct and (R) is the correct explanation of(A)., If both (A) and (R) are correct, but (R) is not the correct explanation of(A)., If(A) is correct, but (R) is incorrect., If both (A) and (R) are incorrect.
View Solution1.0 mole of a monoatomic ideal gas is expanded from state (1) to state (2) as shown in the figure. Calculate ti work done for the expansion of gas from state (1) to state (2) at 298 K

View SolutionWhich of the following species are hypervalent ?
(l) ClO−4
(ll) BF3
(lll) SO2−4
(lV) CO2−3
View SolutionThe heats of formation (ΔH∘f) of the oxides of the third period, sodium to chlorine, are in kJ mol−1
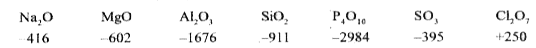
Based on these data, answer the following questions
Which oxide has maximum negative heat of formation per oxygen atom?
View SolutionAssuming that water vapour is an ideal gas, the internal energy change (DeltaU) when 1 mole of water is vaporised at 1 ba r pressure and 100^(@)C, ( given : molar enthalpy of vaporization of water 41kJ mol^(-1) at 1 ba r and 373 K and R=8.3 J mol^(-1)K^(-1)) will be :
View SolutionAssuming that water vapour in an ideal gas, the internal energy change ( DeltaU) when 1 mol of water is vaporised at 1 bar of pressure and 100^(@) C, (Given : Molar enthalpy of vapourization of water at 1 bar and 373 K = 41 kJ mol^(-1) and R=8.3 J mol^(-1)K^(-1) ) will be :
View SolutionAssuming that water vapour is an ideal gas, the internal energy change (ΔU) when 1mol of water is vapourised at 1 bar pressure and 100∘C, (Given: Molar enthalpy of vapourization of water at 1 bar and 373K=41kJmol−1 and R=8.3Jmol−1K−1) will be:
View SolutionAssuming that water vapour is an ideal gas, the internal energy (DeltaU) when 1 mol of water is vapourised at 1 bar pressure and 100^(@)C , (Given : Molar enthalpy of vapourization of water at 1 bar and 373 K = 41 kJ mol^(-1) and R = 8.3 J mol^(-1)K^(-1) ) will be
View SolutionIf water vapour is assumed to be a perfect gas, molar enthalpy change for vaporisation of 1 mol of water at 1 bar and 100∘C is 41 kJ mol−1.Calculate the internal energy change, when
(a) 1 mol of water is vaporised at 1 bar pressure and 100∘C.
(b) 1 mol of water is converted into ice.
View SolutionIf water vapour is assumed to be a perfect gas, molar enthalpy change for vapouration of 1 mole of water at 1 bar and 100^(@)C is 41 kJ mol^(-1) . Calculate the internal energy change when a) 1 mol of water is vapourised at 1 bar and 100^(@)C b) 1 mol of water liquid is converted into ice.
View SolutionAssuming that water vapour is an ideal gas, the internal energy change (DeltaU) when 1 mol of water is vaporised at 1 bar pressure and 100^(@)C , will be (Given: at 1 bar and 373 K, molar enthalpy of vaporisation of water is 41kJ*mol^(-1),R=8.3J*mol^(-1)*K^(-1) )-
View SolutionAssuming that water vapour is an ideal gas, the internal energy change (ΔU) when 1 mol of water is vapourised at 1 bar pressure and 100∘C, (Given : Molar enthalpy of vapourisation of water at 1 bar and 373K=41kJmol−1andR=8.3Jmol−1K−1) will be -
View SolutionIf water vapour is assumed to be a perfect gas, molar enthalpy change for vapourisation of 1 mol of water at 1 bar and 100^(@)C is 41 KJ mol^(-1) . Calculate the internal energy, when i) 1 mol of water is vaporised at 1 bar pressure and 100^(@)C ii) 1 mole of water is converted into ice.
View SolutionIf water vapour is assumed to be a perfect gas, molar enthalpy change for vaporisation of 1 mol of water at 1 bar and 100∘C is 41 kJ mol−1.Calculate the internal energy change, when
(a) 1 mol of water is vaporised at 1 bar pressure and 100∘C.
(b) 1 mol of water is converted into ice.
View SolutionAssuming the water vapour is an ideal gas, the internal energy change (ΔU) when 1 mole of water is vapourised at 1 bar pressure and 100∘C(given molar enthalpy of vapourisation of watyer at 1 bar and 373 K = 41kJmol−1 and R = 8.3 J mol−1K−1) will be
View Solution
