The spontaneity means, having the potential to proceed without the assistance of external agency. The processes which occur spontaneously areSolution in Malayalam
flow of heat from colder to warmer body
gas in a container contracting into one corner
gas expanding to fill the available volume
burning carbon in oxygen to give carbon dioxide
D flowing of heat from warmer to colder body , expanding of gas and burning of carbon to give carbon dioxide, alll are spontaneous process.
Topper's Solved these Questions
- CHEMICAL THERMODYNAMICS BOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL THERMODYNAMICS EXERCISE - LEVEL-II (Assertion-Reason Type)
18 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - QUESTION (LEVEL -ll)
42 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - BRILLIANT PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - QUESTIONS (LEVEL-III ( Linked Comprehension Type ))
12 Videos
Similar Questions
Assertion : Heat of neutralisation of HNO3, and NaOH is almost same as that of HCl and KOH.
Reason : Both HNO3, and HCl are strong acids and NaOH and KOH are strong bases. : if both (A) and (R) are correct and (R) is the correct explanation of(A)., If both (A) and (R) are correct, but (R) is not the correct explanation of(A)., If(A) is correct, but (R) is incorrect., If both (A) and (R) are incorrect.
View SolutionWhich of the following species are hypervalent ?
(l) ClO−4
(ll) BF3
(lll) SO2−4
(lV) CO2−3
View SolutionThe heats of formation (ΔH∘f) of the oxides of the third period, sodium to chlorine, are in kJ mol−1
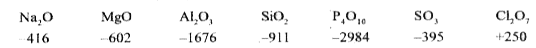
Based on these data, answer the following questions
Which oxide has maximum negative heat of formation per oxygen atom?
View SolutionAll the naturally occurring processes proceed spontaneously in a direction which leads to
View SolutionThe spotanity means having the potential to proceed without assistance of external agency. The processess which occur spontaneously are
View SolutionAssertion (A). Spontaneous process is an irreversible process and may be reversed by some external agency.
Reason (R). Decrease in enthalpy is a contributory factor for spontaneity
View SolutionThe spontaneity means having the potential to proceed without assistance of external agency. The processes which occur spontaneously are
View SolutionAssertion : An exothermic process which is non-spontaneous at high temperature may become spontaneous at low temperature. Reason : Spontaneous process is an irreversible process and may be reversed by some external agency.
View SolutionAll the naturally occuring processes proceed spontaneously in a direction which leads to
View SolutionAll the naturally occurring processes proceed spontaneously in a direction which leads to
View SolutionAssertion (A): Spontaneous process is an irreversible process and may be reversed by external agency Reason (R): Decrease in enthalpy is a contributory factor for spontaneity.
View SolutionThe spontaneity means, having the potential to proceed without the assistance of external agency.The processes which occur spontaneously are
View SolutionAll the naturally occuring processes proceed spontaneously in a direction which leads to
View SolutionAll the naturally occuring processes proceed spontaneously in a direction which leads to
View SolutionAll the naturally occuring processes proceed spontaneously in a direction which leads to
View SolutionThe spontaneity means having the potential to proceed without assistance of external agency. The processes which occur spontaneously are
View Solution

