A heated iron block at 127∘C loses 300 J. of heat to the surroundings which are at a temperature of 27∘.In this process the total change in entropy for system and surroundings is 1xJK−1. Find the value of x.Solution in Malayalam
ΔsysS=qsysTsys=−300273+127=−300400=−34IK−1ΔsysS=−qsysTsurr=−300273+27=300300=+1JK−1
Δ(total)SorΔuniverseS=ΔsysS=ΔsurrS=−34+1=14=0.25JK−1,∴1x=0.25⇒x=10.25=4
টপার এই প্রশ্নগুলো সমাধান করেছে
- CHEMICAL THERMODYNAMICS BOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL THERMODYNAMICS EXERCISE - LEVEL-II (Assertion-Reason Type)
18 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - QUESTION (LEVEL -ll)
42 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - BRILLIANT PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - QUESTIONS (LEVEL-III ( Linked Comprehension Type ))
12 Videos
সম্পর্কিত প্রশ্ন
Assertion : Heat of neutralisation of HNO3, and NaOH is almost same as that of HCl and KOH.
Reason : Both HNO3, and HCl are strong acids and NaOH and KOH are strong bases. : if both (A) and (R) are correct and (R) is the correct explanation of(A)., If both (A) and (R) are correct, but (R) is not the correct explanation of(A)., If(A) is correct, but (R) is incorrect., If both (A) and (R) are incorrect.
View SolutionWhich of the following species are hypervalent ?
(l) ClO−4
(ll) BF3
(lll) SO2−4
(lV) CO2−3
View SolutionThe heats of formation (ΔH∘f) of the oxides of the third period, sodium to chlorine, are in kJ mol−1
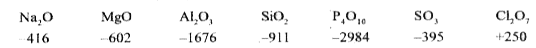
Based on these data, answer the following questions
Which oxide has maximum negative heat of formation per oxygen atom?
View SolutionA heated irron block at 127^(@)C loses 300J of heat to the surroundings which are at a temperature of 27^(@)C . This process is 0.05 J K^(-1) . Find the value of x .
View SolutionAn iron block of mass 2kg, fall form a height 10m. After colliding with the ground it loses 25% enegry to surroundings. Then find the temperature rise of block. (Take sp. Heat of iron 470J/kg∘C)
View SolutionThe entropy change produced in universe when 3.6 kg of water of 47∘ C loses 12 J heat into surrounding at 27∘ C is
View SolutionA heated copper block at 130^(@)C loses 340 J of heat to the surroundings which are at room temperature of 32^(@)C . Calculate (i) the entropy change of the system ( copper block ) (ii) the entropy change in the surrounding (iii) the total entropy change in the universe due to this process Assume that the temperature of the block and the surroundings remains constant.
View SolutionThe total entropy change (DeltaS_("total")) for the system and surrounding of a spontaneous process is given by
View SolutionCalculate the entropy change in the system , and surroundings , and the total entropy changes in the universe during a process in which 245 J of heat flow out of the system 77^(@)C to the surrounding at 30^(@)C.
View SolutionCalculate the entropy change in the system, and in the surroundings and the total entropy change in the universe when during a process 75 J of heat flow out of the system at 55∘C to the surrounding at 20∘C.
View SolutionIn a process, the value of the change in entropy of the system and its surroundings are x and -y J⋅K−1. If x>y, then will the process be spontaneous?
View SolutionA heated copper block at 130^(@)C loses 340 J of heat to the surroundings, which are at room temperature of 32^(@)C . Calculate total entropy change in the universe due to this process.
View SolutionCalculate the entropy change in the system, and in the surroundings and the total entropy change in the universe when during a process 75 J of heat flow out of the system at 55∘C to the surrounding at 20∘C.
View SolutionCalculate the entropy change in the system and surroundings, and the total entropy change in the universe during a process in which 245 J of heat flow out of the system at 77^@C to the surrounding at 33^@C .
View SolutionCalculate the entropy change in the system and surroundings and the total entropy change in the Universe during a process in which 245 J of heat flow out of the system at 77∘C to the surrounding at 33∘C.
View SolutionThe total entropy change (ΔStotal) for the system and surrounding of a spontaneous process is given by
View Solution

