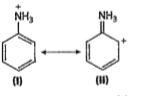
Examine the following structures for the anilinium ion, and choose the correct statement from the ones given below.
 Solution in Malayalam
Solution in Malayalam
II is not an acceptable canonical structure because carbonium ions are less stable than ammonium ions
II is not an acceptable canonical structure because it is not aromatic
II is not an acceptable canonical structure because the nitrogen has 10 valence electrons
II is an acceptable canonical structure
The correct Answer is:A, C
In structure (II), N atom cannot form five bonds (as it violates the normal valency rules) and the positive charge on carbon is less stable than ammonium cation.
টপার এই প্রশ্নগুলো সমাধান করেছে
- NITROGEN COMPOUNDSBOOK - BRILLIANT PUBLICATIONCHAPTER - NITROGEN COMPOUNDSEXERCISE - LEVEL-III (Numerical Type)
5 Videos
- NITROGEN COMPOUNDSBOOK - BRILLIANT PUBLICATIONCHAPTER - NITROGEN COMPOUNDSEXERCISE - LEVEL-III (Matching Column Type)
5 Videos
- NITROGEN COMPOUNDSBOOK - BRILLIANT PUBLICATIONCHAPTER - NITROGEN COMPOUNDSEXERCISE - LEVEL-III (Single Correct Answer Type)
7 Videos
- HALOALKANES AND HALOARENESBOOK - BRILLIANT PUBLICATIONCHAPTER - HALOALKANES AND HALOARENESEXERCISE - Level-II
37 Videos
- NUCLEAR CHEMISTRY BOOK - BRILLIANT PUBLICATIONCHAPTER - NUCLEAR CHEMISTRY EXERCISE - LEVEL-III (Linked Comprehension Type)
11 Videos
সম্পর্কিত প্রশ্ন
The total number of lone-pairs of electrons in melamine is.......
View SolutionMatch the following columns :
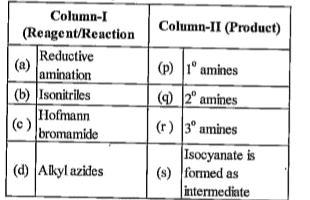
View SolutionAn organic compound (C3H9N) (A), when treated with nitrous acid, gave a alcohol and N2 gas was evolved. (A) on warming with CHCl3 and caustic potash gave (C) which on reduction gave isopropylmethylamine. Predict the structure of(A)
View SolutionWhich is incorrect in the physical properties of halogen compounds ?
View SolutionA sample contains 10−2 kg of two substances A and B with half lives of 4 and 8 sec repsectively (Given that atomic mass of B is twice of A ).
The mass of A and B left after 16 second is :
View SolutionExamine the following two structures for the anilinium ion and choose the correct statement from the ones given below:
View SolutionExamine the following two structures for the anilinium ion and choose the correct statement from the ones gives below:
View SolutionExamine the following two structures for the aniline ion and choose the correct statement from the ones given below
View SolutionExamine the following two structures for the anilinium ion and choose the correct statement from the ones given below :
View SolutionCanonical structures of anilinium ion obtained by accepting a proton are given below. Choose the correct statements .
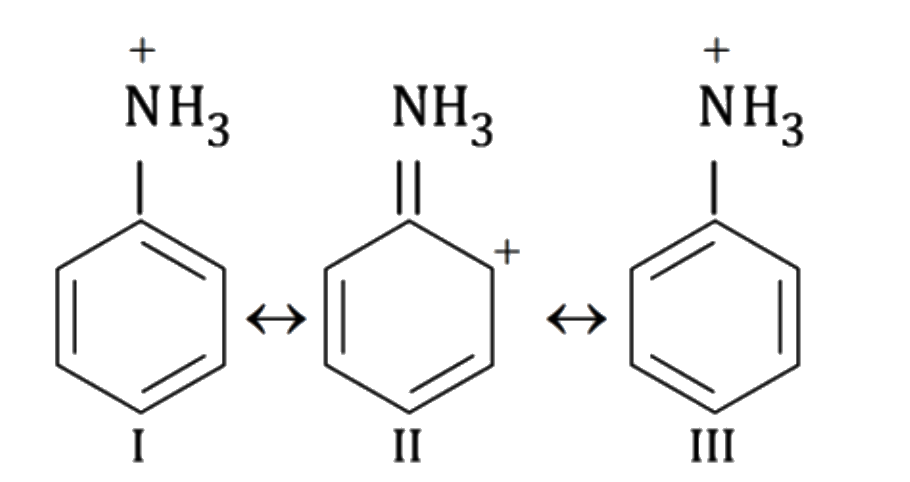
View SolutionPick up the correct statement regarding the following resonating structures of the anilinium ion
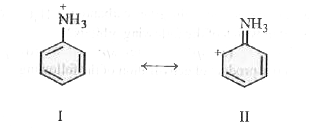
View SolutionExamine the following two structures for the anilinium ion and choose the correct statement from the ones given below :
View SolutionTwo structures of anilinium are given. Choose the correct statement
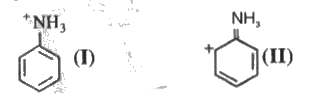
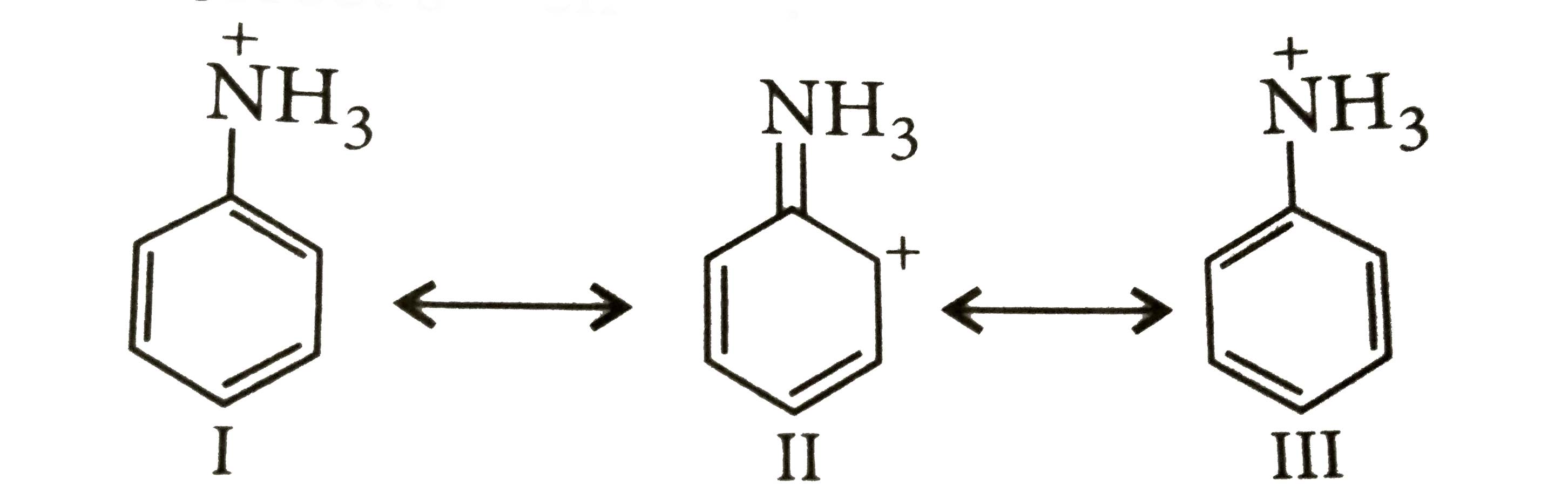
View SolutionCanonical structures of anilinium ion obtained by accepting a proton are given below. Choose the correct statements.
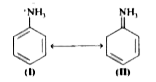
View SolutionChoose the correct statement from the ones given below for the two anilinium ion structures .
View SolutionExamine the following two structures for the anilinium ion and choose the correct statement from the ones given below :
View Solution

