A gas “X” is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of colourless gas "Y". The X and Y respectively, areSolution in Malayalam
CO2,Cl2
Cl2,CO2
Cl2,H2
H2Cl2
The correct Answer is:C
Cl2,H2
Topper's Solved these Questions
- P-BLOCK ELEMENTSBOOK - BRILLIANT PUBLICATIONCHAPTER - P-BLOCK ELEMENTSEXERCISE - LEVEL-II
50 Videos
- NUCLEAR CHEMISTRY BOOK - BRILLIANT PUBLICATIONCHAPTER - NUCLEAR CHEMISTRY EXERCISE - LEVEL-III (Linked Comprehension Type)
11 Videos
- POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE BOOK - BRILLIANT PUBLICATIONCHAPTER - POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE EXERCISE - LEVEL -III (Comprehension Type)
12 Videos
Similar Questions
What may be expected to happen when phosphine gas is mixed with chlorine gas?
View SolutionA sample contains 10−2 kg of two substances A and B with half lives of 4 and 8 sec repsectively (Given that atomic mass of B is twice of A ).
The mass of A and B left after 16 second is :
View SolutionEvery amino acid has a carboxyl group and an amino group, and each group can exist in an acidic form or a basic form depending on the pH of the solution in which the amino acid is dissolved. The carboxyl groups of the amino acids have pKa values of approximately 2, the protonated amino group have pKa values near 9. Therefore, in a very acidic solution (pH near zero), both groups will be in their acidic forms. At a pH of 7, (which is greater than the pKa of the protonated amino group), the carboxyl group will be in its basic form and the amino group will be in its acidic form. In a strongly basic solution (say pH=11), both groups will be in the basic form.
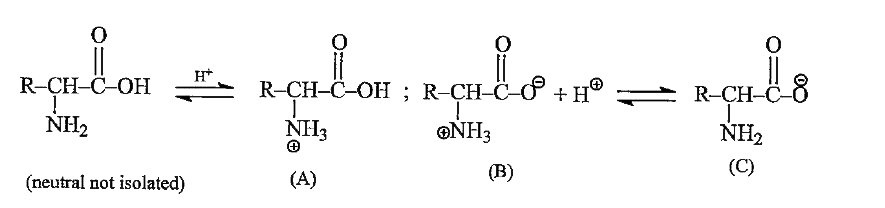
Form A(cationic) of an amino acid exists in
View SolutionA gas X is passed through water to form a saturated solution. The aqueous solution on treatement with AgNO_(3) gives a white precipitate. The saturated aqueous solution also dissolves Mg ribbon with evolution of colourless gas Y,X and Y are respectively:
View SolutionA gas X is passed through water to forms a saturated solution .The aqaeous solution on tretment aqeous solution also dissolve magnassium ribbon with the evolation of a colourless gas Y identify X and Y
View SolutionA gas X is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of a colourless gas Y. Identify X and Y.
View SolutionA gas 'X' is passed through water to form a solution. The aqeous solution on treatment with AgNO3 solution gives a white precipitate. The sturated aqueous solution also dissolves magnesium ribbon with the evolution of colourless gas 'Y'. Identify 'X' and 'Y'
View SolutionA greenish yellow gas 'X' is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate solution gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with the evolution of a colourless gas 'Y'. Identify gases 'X' and 'Y'.
View SolutionA gas X is passed through water to form a saturated solution. The aqueous solution on treatement with silver nitrate give a white precipitate. The saturated aqueous solution dissolve magnesium ribbon with evolution of a colourless gas 'Y'. Identify X and Y
View SolutionA gas 'X' is passed through water to form a saturated solution. The aqueous solution on treatement with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of a colourless gas 'Y' Identify 'X' and 'Y'
View SolutionA gas 'X' is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of a colourless gas 'Y'. The compound 'X' is treated with hot concentrated NaOH, a compound Z is formed. The average bond order between Cl and O atom in (Z) is ________.
View SolutionA greenish yellow gas ‘X’ is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate solution gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with the evolution of a colourless gas ‘Y’. Identify gases ‘X’ and ‘Y’.
View SolutionA gas ‘X' is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of a colourless gas 'Y’: Identify ‘X' and 'Y’.
View Solutionएक गैस 'X ' को जल में प्रवाहित करके एक संतृप्त विलयन बनाया जाता है। इस जलीय विलयन को सिल्वर नाइट्रेट के साथ अभिकृत कराने पर एक सफ़ेद अवक्षेप प्राप्त होता है। संतृप्त जलीय विलयन, मैग्नीशियम रिबर को विलेय करके एक रंगहीन 'Y ' उत्पन्न करता है। 'X ' तथा 'Y ' की पहचान कीजिए।
View SolutionA gas 'X' is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of colourless gas Y , X and Y are :
View SolutionA gas 'X' is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate gives a white ppt. The saturated aqueous solution also dissolve magnesium ribbon with evolution of a colourless gas 'Y'. Identify 'X' and 'Y'.
View Solution

