An optically active compound C6H12O gives positive test with 2, 4-dinitrophenyl hydrazine, but negative with Tollens' reagent, what is the structure of the compound ?
CH3−O∣∣C−CH2−CH2−CH2−CH3
H−O∣∣C−CH∣CH3−CH2−CH2−CH3
CH3−O∣∣C−CH∣CH3−CH2−CH3
CH3−CH2−O∣∣C−CH∣CH3−CH3
The correct Answer is:C
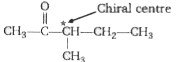 2,4-DNP is test for aldehyde and ketone
2,4-DNP is test for aldehyde and ketone
Similar Questions
Compound C4H8CI2(A) on hydrolysis gives a compound C4H8O(B) which reacts with hydroxylamine and does not give any test with Tollens reagent . What are (A) and (B) ?
View SolutionAn compound (A ) C4H8Cl2 on alkaline hydrolysis to give compound (B ) C4H8O which gives an oxime and positive Tollen's reagent test . What is the structure of (A) ?
View SolutionC(C6H12), and optically active dydrocarbon which on catalytic hydrogenation gives an optically inactive compound, C6H14.
View SolutionA compound C5H10O(A) forms a phenylhydrazone and gives negative Tollens' test and a positive iodoform reaction. It gives n-pentane on reduction. The compound (A) is
View SolutionAn optically active compound A (C6H12O)gives positive test with 2,4-D NP, but negative test with Tollen's reagent. Compound A is
View Solution(A) A(C7H14)O3/Red−−−−→B(C3H6O)+C
(B) Gives positive Tollens test but negative iodform test.
(C ) Give negative Tollens test but positive iodoform test.
The compound (A) is:
View Solution(A) A(C_(7)H_(14)) overset(O_(3)//Red)(rarr)B(C_(3)H_(6)O)+C (B) Gives positive Tollens test but negative iodform test. (C ) Give negative Tollens test but positive iodoform test. The compound (B) is:
View Solution(A) A(C7H14)O3/Red−−−−→B(C3H6O)+C
(B) Gives positive Tollens test but negative iodform test.
(C ) Give negative Tollens test but positive iodoform test.
The compound (C ) is:
View Solution

