Which of the following statement are correct ? Solution in Malayalam
The increasing order of osmotic pressure of 0.1M aqueous solution is as follows 0.1M Glucose <0.1M Sodium chloride <0.1M Magnesium chloride.
Density of a solution in independent of temperature
Camphor has comparatively larger value of Kf as compared to other common solvents
Limiting value of van't Hoff factor of K4[Fe(CN)6] is 11.
The correct Answer is:A, C
(A) The total concentration of species of MgCl2 solution will be 0.3M and in NaCl solution it will be 0.2 M. Glucose in a nonelectrolyte, hence concentration of glucose solution will be 0.1M
(B) Density is defined as mass per unit volume and the volume of the solution varies with temperature
(D) In solution, K4Fe(CN)6 ionizes as K4Fe(CN)6⇔4k++Fe(CN)4−6. Hence, the limiting vlaue of vant.s Hoff factor will be 5.
Topper's Solved these Questions
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level-III (Numerical Type)
Explore 8 Videos
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level-III (Matching Column Type)
Explore 5 Videos
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level -III (Single Correct Answer Type)
Explore 8 Videos
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3 (Linked Comprehension Type)
Explore 10 Videos
- STEREOCHEMISTRY Book:BRILLIANT PUBLICATIONChapter:STEREOCHEMISTRY Exercise:LEVEL-II (ASSERTION - REASON TYPE)
Explore 2 Videos
Similar Questions
The mass of glucose that should be dissolved in 50g of water in order to produce the same lowering of vapour pressure as is produced by dissolving 1g of urea in the same quantity of water is
View SolutionMatch the columns
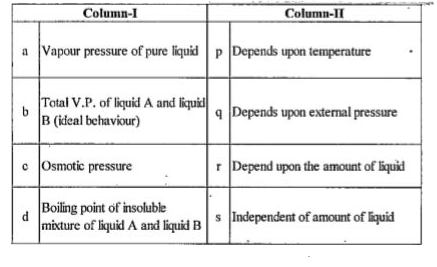
View SolutionTwo components A and B form an ideal solution. The mole fractions of A and B in solution are XAandXB, while that in vapour phase are YAandYB. The slope and intercept of plot of 1YA vs 1XA will be
View SolutionF centre is an example of metal excess defect due to the presence of extra cations at interstitial sites. Zinc oxide is white in colour at room temperature, on heating it loses oxygen and turns yellow.
ZnOHeat−−→Zn2++12O2+2e−
Now there is excess of zinc in the crystal and its formula becomes Zn1+x O. the excess of Zn2+ ions move to interstitial sites and the electrons to neighbouring interstitial sites.
There are many solids which are difficult to prepare in the stiochiometric composition and contain less amount of the metal as compared to stiochiometric proportion. a typical example of this type is FeO which is mostly found with a composition of Fe0.95. it may actually range from Fe0.96 O.
Q. In Fe0.95 O:
View SolutionAssertion : Meso tartaric acid is optically inactive
Reason : Meso tartaric acid has a plane of symmetry
View SolutionWhich of the following statements is not correct ?
View SolutionWhich of the following statements are correct statements ?
View SolutionWhich of the following statement is correct .
View SolutionWhich of the following statements is not correct?
View SolutionWhich of the following statement is correct?
View SolutionWhich of the following statement is /are correct ?
View SolutionWhich of the following statements is correct?
View SolutionWhich of the following is/are correct statement/statements ?
View SolutionWhich of the following statement is not correct?
View SolutionWhich of the following statements is correct ?
View SolutionWhich of the following statements are correct ?
View Solution
