1 mole of A and 1 mole B are mixed together to form an ideal solution at 25∘C. Solution is placed in a vessel fitted with piston. If pressure on the solution is reduced isothermally.
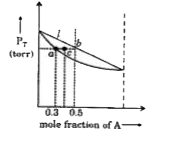
Correct relationship between P∘AandP∘B is
3P∘A=7P∘B
P∘A>P∘B
P∘A<P∘B
3P∘B=7P∘A
The correct Answer is:D
yAyB=p∘AxAP∘BxB⇒0.30.7=P∘AP∘B×0.50.5
⇒3P∘B=7P∘B
Topper's Solved these Questions
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level-III (Statement Type)
Explore 6 Videos
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3 (Linked Comprehension Type)
Explore 10 Videos
- STEREOCHEMISTRY Book:BRILLIANT PUBLICATIONChapter:STEREOCHEMISTRY Exercise:LEVEL-II (ASSERTION - REASON TYPE)
Explore 2 Videos
Similar Questions
One mole of component P and two moles of component Q are mixed at 27∘C to form an ideal binary solution. Select the correct option for given information?
View Solution1 mole heptane (V.P=92mmofHg) is mixed with 4 mol. Octane (V.P=31 mm of Hg), form an ideal solution. Find out the vapour pressure of solution.
View Solution
Knowledge Check
One mole of component P and two moles of component Q are mixed at 27∘C to form an ideal binary solution. Select the correct option for given information?
AΔSmix=3.82calk−1BΔHmix=0CΔSmix=0DΔGmax=−1145.7 cal20 moles of liquid A are mixed with 20 moles of liquid B to form an ideal binary solution. Calculate moles of A in liquid state when half of the solution has vaporized :
( Given : P∘A=100 torr,P∘B=121 torr)A1121B11021C20021D2202110 mole of liquid 'A' and 10 mole of liquid 'B' is mixed in a cylindrical vessel containing a piston arrangement. Initially a pressure of 2 atm is maintained on the solution. Now, the piston is raised slowly and isothermally. Assuming A and B to be completely miscible and forming an ideal solution.
P∘A=0.6 atm and P∘B=0.9 atm
If 14 of the total amount of liquid solution taken initially is converted to vapour, then moles of A in the vapour will be :
[Given : √2425=49.25]A2.125B3.875C4D1
1 mole heptane (V.P=92mmofHg) is mixed with 4 mol. Octane (V.P=31 mm of Hg), form an ideal solution. Find out the vapour pressure of solution.
View Solution1 mole heptane (V.P=92mmofHg) is mixed with 4 mol. Octane (V.P=31 mm of Hg), form an ideal solution. Find out the vapour pressure of solution.
View Solution10 mole of liquid 'A' and 10 mole of liquid 'B' is mixed in a cylindrical vessel containing a piston arrangement. Initially a pressure of 2 atm is maintained on the solution. Now, the piston is raised slowly and isothermally. Assuming A and B to be completely miscible and forming an ideal solution.
P∘A=0.6 atm and P∘B=0.9 atm
The minimum pressure for the existance of liquid solution is :
View Solution10 mole of liquid 'A' and 10 mole of liquid 'B' is mixed in a cylindrical vessel containing a piston arrangement. Initially a pressure of 2 atm is maintained on the solution. Now, the piston is raised slowly and isothermally. Assuming A and B to be completely miscible and forming an ideal solution.
P∘A=0.6 atm and P∘B=0.9 atm
The pressure below which the evaporation of liquid solution will start is :
View SolutionLiquid A and B form an ideal solution. The boiling point of solution is 72∘ C, when the external pressure is 0.6 bar. If then solution contains 200 moles of liquid A, then moles of liquid B is :
[Given:P∘A=0.4barandP∘B=1.0barat72∘C]
View Solution

