If the intensity of light is increased for the same colour?
frequency will increase
no. of photons will increase
KE of photoelectrons will increase
momentum will increase
To solve the question regarding the effect of increasing the intensity of light for the same color, we need to analyze the relationship between intensity, number of photons, frequency, kinetic energy of photoelectrons, and momentum. Here’s a step-by-step breakdown:
1. Understanding Intensity:
- Intensity (I) of light is defined as the power per unit area. It can also be understood as the energy delivered per unit time per unit area.
2. Relation Between Intensity and Number of Photons:
- The intensity of light is directly proportional to the number of photons hitting a surface. This can be expressed mathematically as:
I∝n
where n is the number of photons.
3. Effect of Increasing Intensity:
- When the intensity of light is increased while keeping the color (and hence the frequency) constant, the number of photons incident on the surface increases. Thus, we can conclude:
If intensity increases, number of photons increases.
4. Frequency of Light:
- The frequency of light is a characteristic of the light itself and is determined by its color. Increasing the intensity does not change the frequency of the light. Therefore:
Frequency remains constant.
5. Kinetic Energy of Photoelectrons:
- The kinetic energy (KE) of the emitted photoelectrons is given by the equation:
KE=E−W
where E is the energy of the photon (which depends on frequency) and W is the work function of the metal. Since the frequency (and thus the energy per photon) remains constant, the kinetic energy of the photoelectrons does not increase with intensity. Therefore:
Kinetic energy remains constant.
6. Momentum of Photons:
- The momentum p of a photon is given by:
p=Ec=h⋅fc
where h is Planck's constant, f is the frequency, and c is the speed of light. Since the frequency does not change with intensity, the momentum of individual photons also remains constant. Thus:
Momentum remains constant.
7. Conclusion:
- From the above analysis, we conclude that increasing the intensity of light for the same color results in an increase in the number of photons, while the frequency, kinetic energy of photoelectrons, and momentum of the photons remain unchanged.
Final Answer:
- If the intensity of light is increased for the same color, the number of photons will increase.
- Intensity (I) of light is defined as the power per unit area. It can also be understood as the energy delivered per unit time per unit area.
- The intensity of light is directly proportional to the number of photons hitting a surface. This can be expressed mathematically as:
I∝n
where n is the number of photons.
- When the intensity of light is increased while keeping the color (and hence the frequency) constant, the number of photons incident on the surface increases. Thus, we can conclude:
If intensity increases, number of photons increases.
- The frequency of light is a characteristic of the light itself and is determined by its color. Increasing the intensity does not change the frequency of the light. Therefore:
Frequency remains constant.
- The kinetic energy (KE) of the emitted photoelectrons is given by the equation:
KE=E−W
where E is the energy of the photon (which depends on frequency) and W is the work function of the metal. Since the frequency (and thus the energy per photon) remains constant, the kinetic energy of the photoelectrons does not increase with intensity. Therefore:
Kinetic energy remains constant.
- The momentum p of a photon is given by:
p=Ec=h⋅fc
where h is Planck's constant, f is the frequency, and c is the speed of light. Since the frequency does not change with intensity, the momentum of individual photons also remains constant. Thus:
Momentum remains constant.
- From the above analysis, we conclude that increasing the intensity of light for the same color results in an increase in the number of photons, while the frequency, kinetic energy of photoelectrons, and momentum of the photons remain unchanged.
- If the intensity of light is increased for the same color, the number of photons will increase.
Topper's Solved these Questions
- JEE MAIN 2021BOOK - JEE MAINS PREVIOUS YEARCHAPTER - JEE MAIN 2021EXERCISE - SECTION-A
80 Videos
- JEE MAIN 2021BOOK - JEE MAINS PREVIOUS YEARCHAPTER - JEE MAIN 2021EXERCISE - SECTION-B
40 Videos
- JEE MAINBOOK - JEE MAINS PREVIOUS YEARCHAPTER - JEE MAINEXERCISE - All Questions
473 Videos
- JEE MAIN 2022BOOK - JEE MAINS PREVIOUS YEARCHAPTER - JEE MAIN 2022EXERCISE - Question
492 Videos
Similar Questions
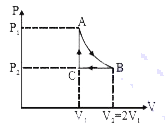
n mole a perfect gas undergoes a cyclic process ABCA (see figure) consisting of the following processes.
A→B: Isothermal expansion at temperature T so that the volume is doubled from V1 to V2=2V1 and pressure changes from P1 to P2
B→C : Isobaric compression at pressure P2 to initial volume V1
C→A : Isochoric change leading to change of pressure from P2 toP1 .
Total work done in the complete cycle ABCA is :
View SolutionThe coefficient of static friction between a wooden block of mass 0.5 kg and a vertical rough wall is 0.2. The magnitude of horizontal force that should be applied on the block to keep it adhere to the wall will be ____N.
View SolutionIf weight of an object at pole is 196 N then weight at equator is [g=10ms2 , radius of earth = 6400 Km]
View SolutionThe normal reaction 'N' for a vehicle of 800 kg mass, negotiating a turn on a 30∘ banked road at maximum possible speed without skidding is _______ ×103 kg m/s2.
View SolutionAs the intensity of incident light increases
View SolutionWhen the intensity of a light source is increased
View SolutionAssertion(A): The kinetic of the photo electron ejected increases with increases in intensity of incident light.
Reason(R): Increase in intensity of incident light increases the rate of emission.
View Solutionजब प्रकाश की तीव्रता को बढ़ाया जाता है, तो
View SolutionIn a photocell, increasing the intensity of incident light increases "……………….."
View SolutionWhen the intensity of a light source is increased.
View SolutionWhen intensity of incident light increases
View Solutionआपतित प्रकाश की तीव्रता बढ़ाने पर
View SolutionAssertion : The increase in intensity of incidence light decreases the kinetic energy of emitted electrons. Reason : When frequency of light increases , the intensity of light increases.
View SolutionAs the intensity of incident light increases
View SolutionAs the intensity of incident light increases
View SolutionIf the intensity of the image light increases
View Solution

