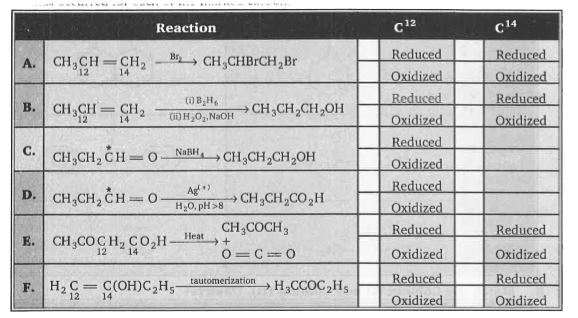
Consider reactions A through F. Those carbon atoms undergoing change, as part of a functional group, are marked as C12,C14 or starred. In the cases shown, each carbon atom has either been reduced or oxidized. Your job is to identify the change in oxidation state that has occurred for each of the marked carbon.
 Solution in Kannada
Solution in Kannada
The correct Answer is:A : both are oxidized; B : C12 is reduced, C14 is oxidized; C : reduced; D : oxidized; E : C12 is reduced, C14 is oxidized; F : C12 is reduced, C14 is oxidized
A : both are oxidized , B : C12 is reduced, C14 is oxidized , C : reduced , D : oxidized , E : C12 is reduced, C14 is oxidized , F : C12 is reduced, C14 is oxidized
Topper's Solved these Questions
- ALDEHYDES AND KETONESBOOK - MS CHOUHANCHAPTER - ALDEHYDES AND KETONESEXERCISE - LEVEL-2
16 Videos
- ALCOHOL,ETHERS AND EPOXIDESBOOK - MS CHOUHANCHAPTER - ALCOHOL,ETHERS AND EPOXIDESEXERCISE - SUBJECTIVE PROBLEMS
1 Video
- ALKYL HALIDES (ELIMINATION REACTION) BOOK - MS CHOUHANCHAPTER - ALKYL HALIDES (ELIMINATION REACTION) EXERCISE - LEVEL-2
23 Videos
Similar Questions
The following questions refer to the compounds (A to G) shown below :
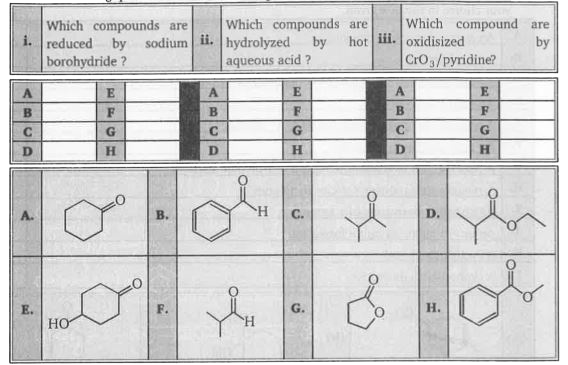
View Solution
Maximum number of moles of Ac2O consumed by reactant (A) is :
View SolutionComprehension
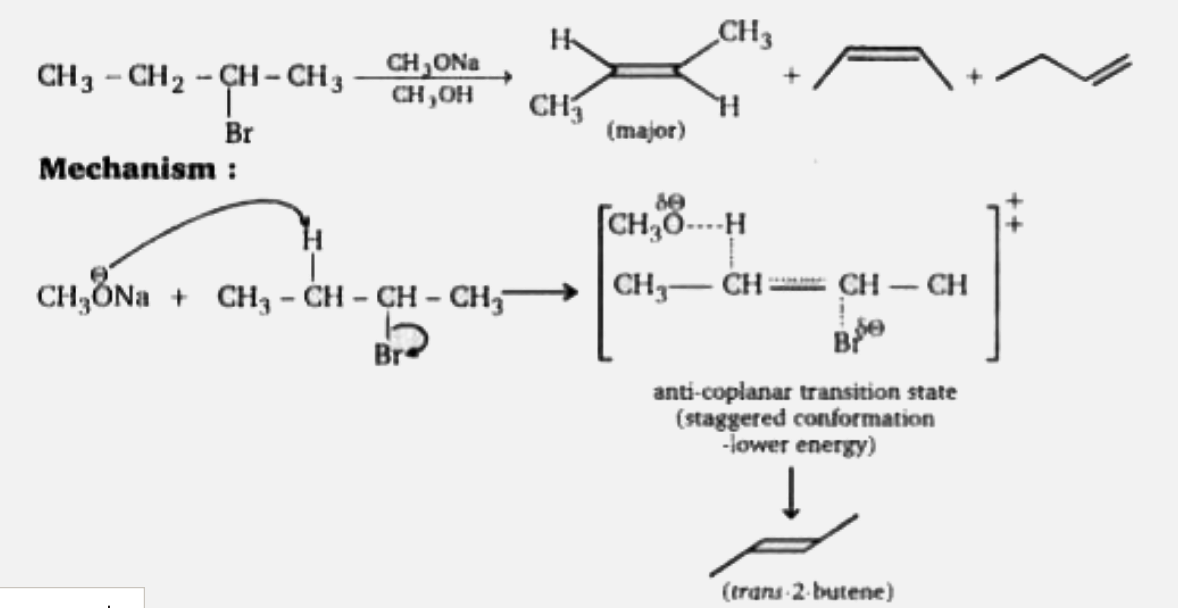
E2 raction → Elimination bimolecular
In the general mechanism of the E2 reaction a strong base abstract a proton on a carbon atom adjacent to the one of the leaving group. As the abstracts a proton , a double bond forms and the leaving group leaves.
Identify the rate of reaction of given compounds in E2 reaction:
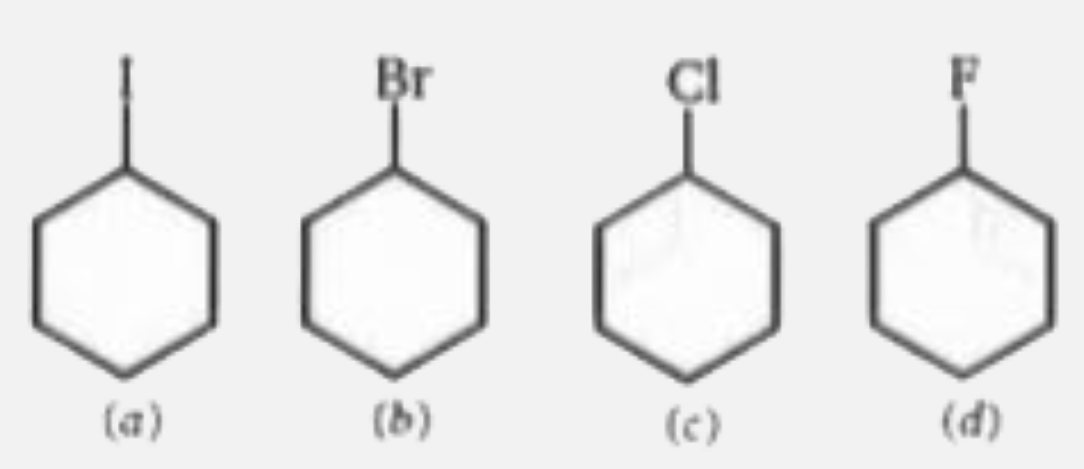
View SolutionWhat is the type of hybridisation of carbon atoms marked with star?
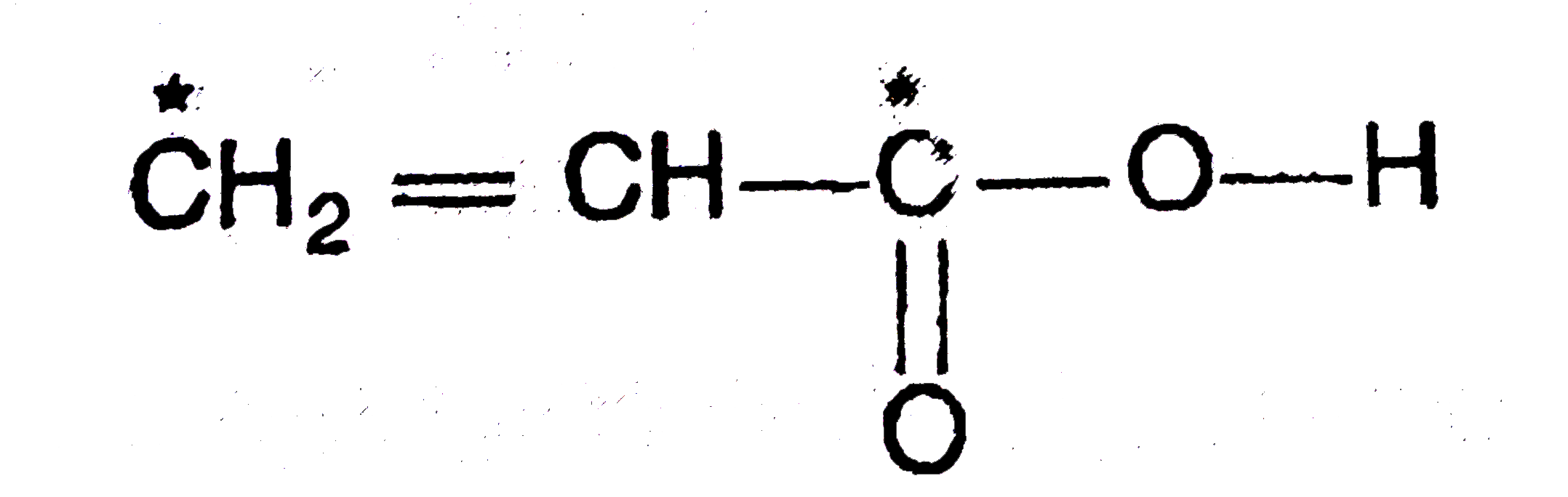
View SolutionOxidation states of carbon atoms in diamond and graphite are.
View SolutionIdentify (A) to (F) and mark the C⋆ carbon in the entire scheme :
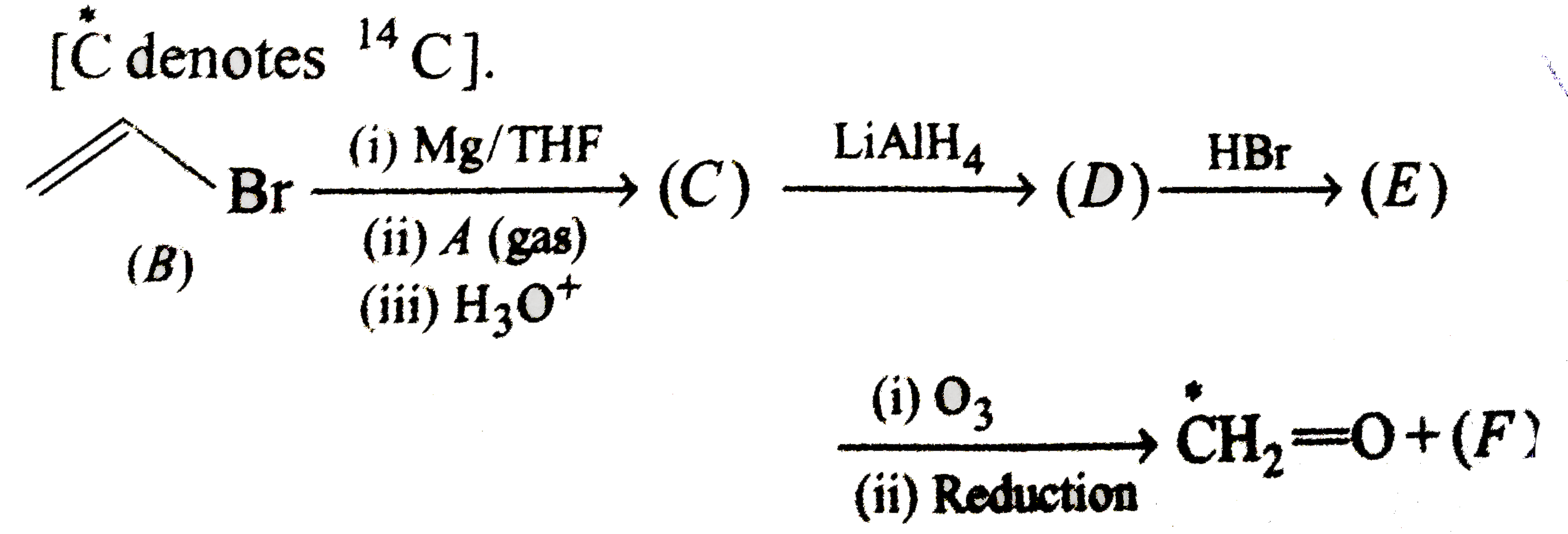
View SolutionPredict the oxidation state of carbon in each of the following compounds. C Cl_(4)
View SolutionIn benzene, each carbon atom undergoes :
View SolutionIn benzene, each carbon atom undergoes
View SolutionOxidation states of carbon atoms in methane and ethane are :
View SolutionIndicate the hybrid state of each carbon atom in compounds - CH_2 = C = O
View SolutionCH_3CONH_2 rarrCH_3CN The hybridization of the carbon atoms of the functional group in this conversion changes-
View SolutionCH_3CONH_2 rarrCH_3CN এই রূপান্তরীকরণে কার্যকরী গ্রুপের কার্বন পরমাণুর সংকরায়ন অবস্থার পরিবর্তন-
View SolutionIn the carbonyl group, the carbon atom undergoes
View Solutionकार्बन-मोनो ऑक्साइड के एक अणु में कार्बन तथा ऑक्सीजन परमाणुओं के बीच की दूरी 1.1Å है। दिया है, कार्बन परमाणु का द्रव्यमान 12a.m.u. एवं ऑक्सीजन परमाणु का द्रव्यमान 16a.m.u. है। कार्बन मोनोक्साइड के द्रव्यमान केन्द्र की स्थिति है
View SolutionCarbon-12 atoms has ______.
View Solution

