57 of a number is 23 . What is the number ?
A)1514
B)157
C)715
D)1415
1514
157
715
1415
The correct Answer is:D
To solve the problem, we need to find the number x such that 57 of x is equal to 23.
1. Set up the equation:
We start by expressing the relationship given in the problem. We can write:
57×x=23
2. Isolate x:
To find x, we can multiply both sides of the equation by the reciprocal of 57, which is 75:
x=23×75
3. Multiply the fractions:
Now, we multiply the fractions on the right side:
x=2×73×5=1415
4. Final answer:
Thus, the required number x is:
x=1415
Conclusion:
The answer is 1415, which corresponds to option D.
We start by expressing the relationship given in the problem. We can write:
57×x=23
To find x, we can multiply both sides of the equation by the reciprocal of 57, which is 75:
x=23×75
Now, we multiply the fractions on the right side:
x=2×73×5=1415
Thus, the required number x is:
x=1415
The answer is 1415, which corresponds to option D.
Similar Questions
Find correct features w.r.t. most advanced type of placentation. Br a. Parietal in Argemone. Br b. Basal in cereals. Br c. Axile in solanaceae. Br d. Unilocular ovary. Br e. Ovary with single ovule
View SolutionWite down the corresponding elements in the figure : 1)……………… br 2)…………… br 3).……………. Br 4)………….. Br 5)…………. Br 6)…………..
View Solutionमान निकालिए : 157+(−514)
View SolutionFind the difference:
1415−715
View SolutionConsider the following modified structures in plants and select ontogenetically similar structures, br A. Phyllode in Acacia, br B. Thorn in Citrus, br C. Tendril in watermelon, br D. Cladode in Asparagus, br E. Spines in cacti
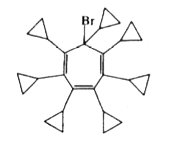
View SolutionIn the given compound, will Br ionize in the form of (a) Br^(-) or (b) Br^(+) .
View SolutionIn the given compound, will Br ionize in the form of (a) Br− or (b) Br+.
View SolutionOxidation state of Br in Br2andBrO−3 is
View Solution