Account for the trend in melting points of 3d series.
The melting point first increases as the number of unpaired .d. electrons available for metallic bonding increases, reach a maximum and then decreases, as the .d. electrons pair up and becomes unavailable for metallic bonding. The maximum melting point is in the middle, where the transition metal has d5 configuration. This results in the formation of strong metallic bonding. Which results in the maximum melting point in the middle of the series.
Topper's Solved these Questions
- TRANSITION AND INNER TRANSITION ELEMENTS BOOK - PREMIERS PUBLISHERSCHAPTER - TRANSITION AND INNER TRANSITION ELEMENTS EXERCISE - OTHER IMPORTANT QUESTIONS & ANSWERS (I. Choose the correct answer)
27 Videos
- SURFACE CHEMISTRYBOOK - PREMIERS PUBLISHERSCHAPTER - SURFACE CHEMISTRYEXERCISE - OTHER IMPORTANT QUESTIONS & ANSWERS (II. Answer the following questions:)
57 Videos
Similar Questions
The correct order of decreasing second ionisation enthalpy of Ti(22), V(23), Cr(24) and Mn(25) is :
View SolutionGive three examples of adsorption
View SolutionWhy Zinc has lowest melting point in 3d series?
View SolutionThe melting point of Zn is lower as compared to those of the other elements of 3d series because:
View Solution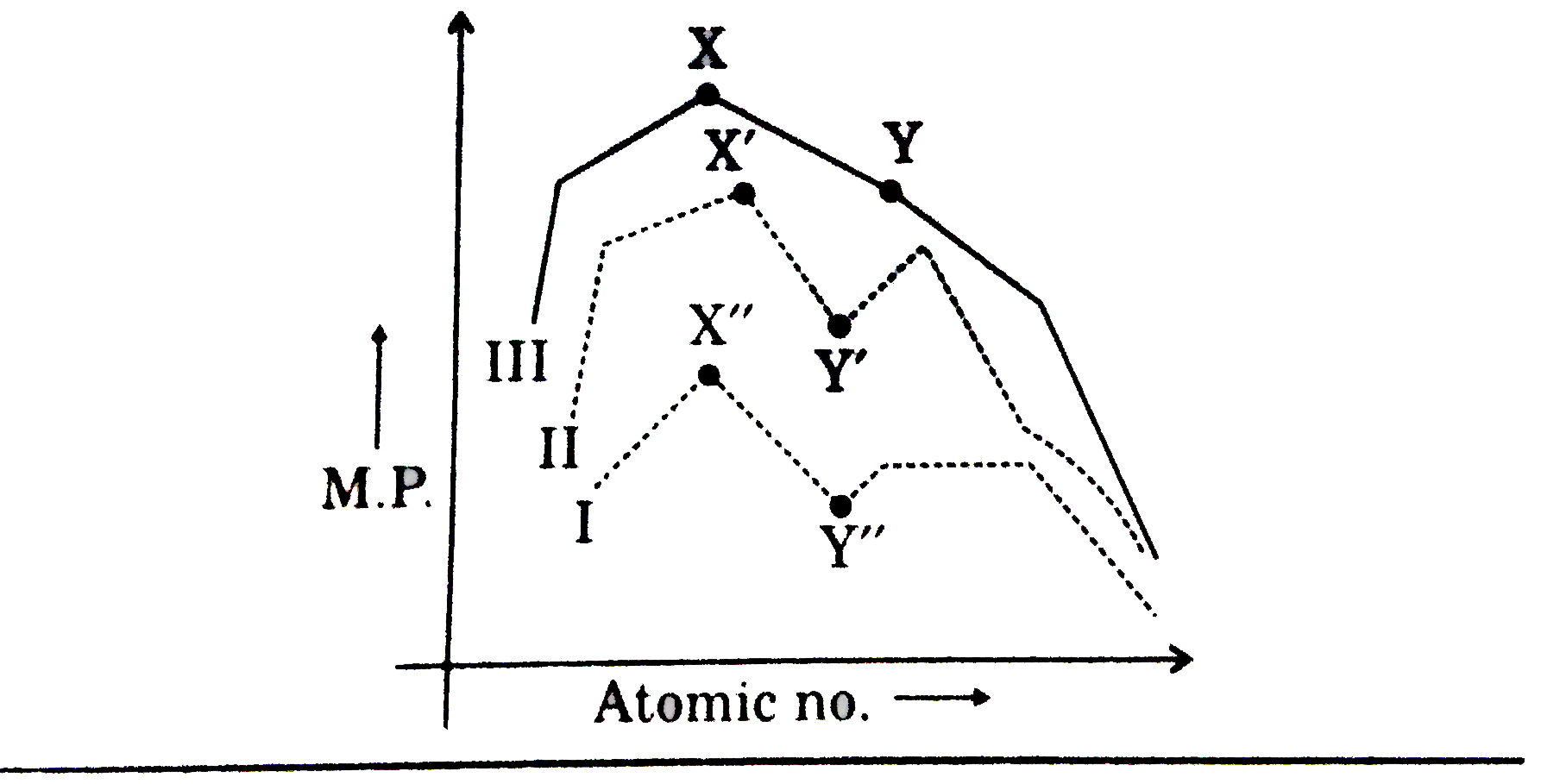
Refer to the graph for trends in M.P. of transition elements of 3d,4d and 5d series.
Q. A metal having high boiling point:
View SolutionWhich of the following transition metals of 3d series has the lowest melting point ?
View SolutionAmong the first transition series (3d series) Chromium has highest melting point. Why?
View SolutionAssertion : Mn shows exceptional melting point in 3d series. Reason : Its outer configuration is 4s^(2) 3d^(5)
View SolutionWrite the trends : Melting points of the transition elements in each series.
View SolutionA) In a group the correct order of melting points is 3d<4d<5d
B) VI B group have highest melting points in any series
C) In 3d and 4d series, VIIB group elements have exceptionally low melting points
D) The correct order of melting points is Cu>Ag>Au
View SolutionIn 3d series, the elements which exhibit highest and lowest melting point are respectively
View SolutionGive melting point trend in hydride of Group 17
View SolutionIn 3d series which element has highest melting point
View SolutionIdentify the correct statements
(a) In a group the correct order of melting points is 3d < 4d < 5d
(b) In 3d and 4d series, VII B group elements have exceptionally low melting points
(c) II B group have highest melting points in any series.
(d) The correct order of melting points is Cu > Ag > Au
View SolutionAccount for the following trends in atomic and ionic radii of transition metals. The atomic radii of elements in 4d series are more than that of corresponding elements in 3d series.
View SolutionHighest melting point among the 3d block element.
View Solution

