Which of the following would be the best (most reactive) nucleophile in the polar medium ?
I−
Br−
Cl−
F−
The correct Answer is:A
A polar protic solvent can participate in hydrogen bonding with a nucleophile. This make the nucleophile less reactive, as its lone pair becomes less available to attack. The ability of nucleophiles to participate in hydrogen bonding decreases as we go down the periodic table. This means that the lone pairs of iodide ion will be considerably more "free" than those of fluoride, resulting in higher rates (and greater nucleophilicity).
Topper's Solved these Questions
Similar Questions
The correct IUPAC name of the following hydrocarbon is
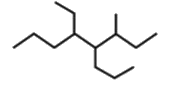
View SolutionThe radius ratio of KF is 0.98. The structure of KF is of the type
View SolutionWhich of the following alkoxides is the most reactive nucleophile?
View SolutionThe most reactive nucleophile among the following is :
View SolutionThe most reactive nucleophile among the following is
View SolutionThe most reactive nucleophile among the following is
View Solutionwhich is more reactive nucleophile in polar protic solvent ?
View SolutionWhich is more reactive nucleophile in polar aprotic solvent ?
View SolutionWhich among the following is most reactive in nucleophilic addition ?
View SolutionThe most reactive nucleophile among the following is
View SolutionWhich of the following would be the best (most reactive) nucleophile in the polar medium?
View SolutionThe most reactive nucleophile among the following is :
View SolutionWhich one of the following is most reactive in nucleophilic substitution ?
View SolutionWhich of the following is most reactive to give nucleophilic addition?
View Solutionनिम्नलिखित में सै कौन सा नाभिकस्नेही ध्रुवीय माध्यम में सबसे अच्छा (सबसे अधिक क्रियाशील) नाभिक स्नेही होगा ?
View Solutionwhich is more reactive nucleophile in polar protic solvent ?
View Solution

