Which of the following is not correct?
he metallic conduction is due to the movement of electrons in the metal
The electrolytic conduction is due to the movement of ions in the solution
The current carrying ions are not necessarily discharged at the electrodes
The metallic conduction increases with the increase in temperature, whereas that of electrolytic conduction decreases with temperature.
The correct Answer is:D
The metallic conduction decreases with increase in temperature, but electrolytic conduction increases with the increase in temperature. Because the metallic conduction is due to the movement of electrons in the metal and the electrolytic conduction is due to the movement of ions in the solution.
Topper's Solved these Questions
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES TEST Book:NTA MOCK TESTSChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES TEST Exercise:SINGLE CHOICE
Explore 30 Videos
- ENVIRONMENTAL CHEMISTRY TESTBook:NTA MOCK TESTSChapter:ENVIRONMENTAL CHEMISTRY TESTExercise:CHEMISTRY
Explore 30 Videos
Similar Questions
The set representing the correct order of ionic radii is
View SolutionEutrophication causes reduction in
View SolutionWhich of the following statements is/are correct about the following is/are correct about the following reactions ?
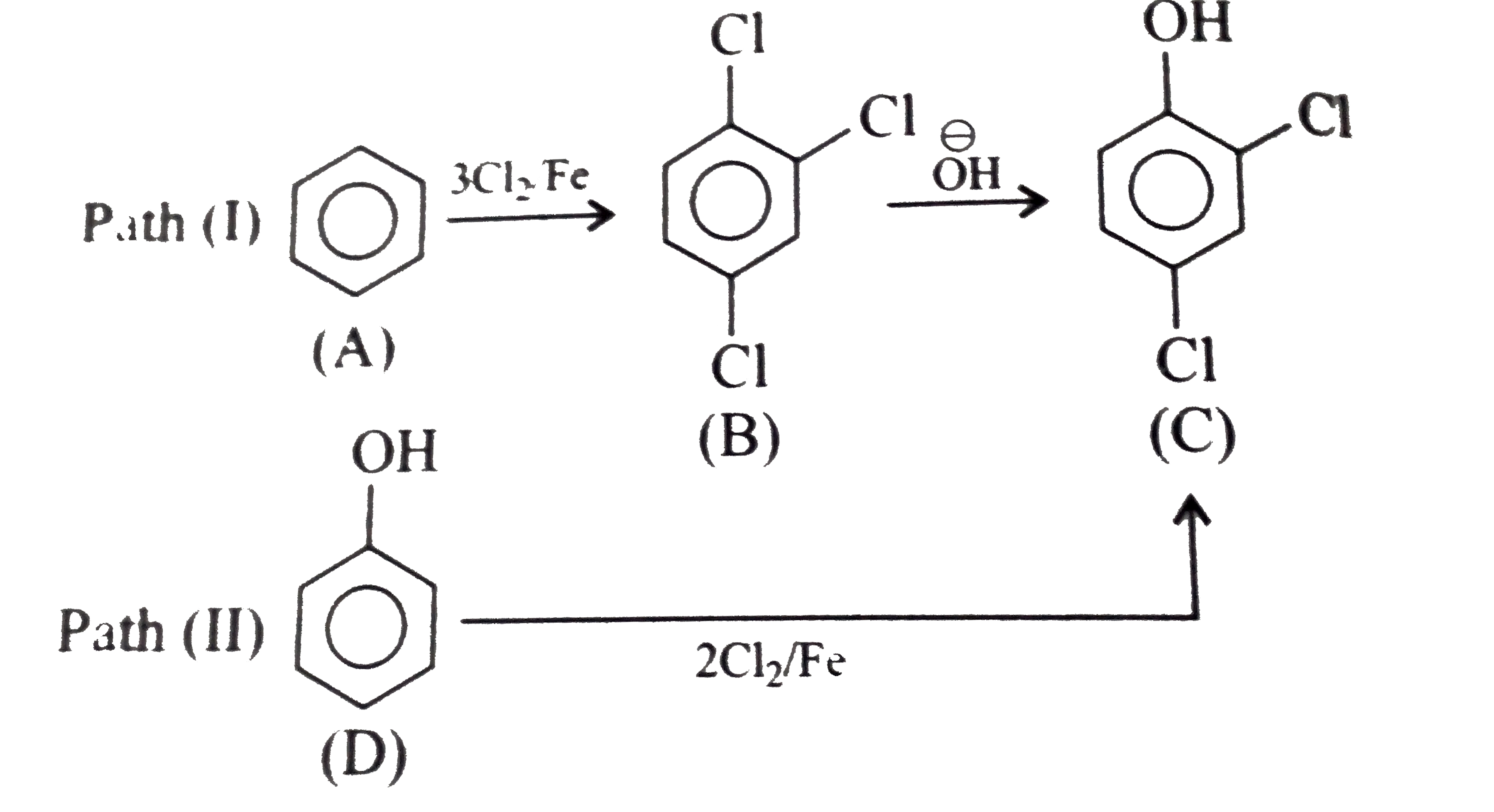
View SolutionWhich of the following statements are correct?
View SolutionWhich of the following is not correct?
View Solutionगेटो के निम्न संयुग्मन के लिए कौन-सी सत्यता सारणी सही है -
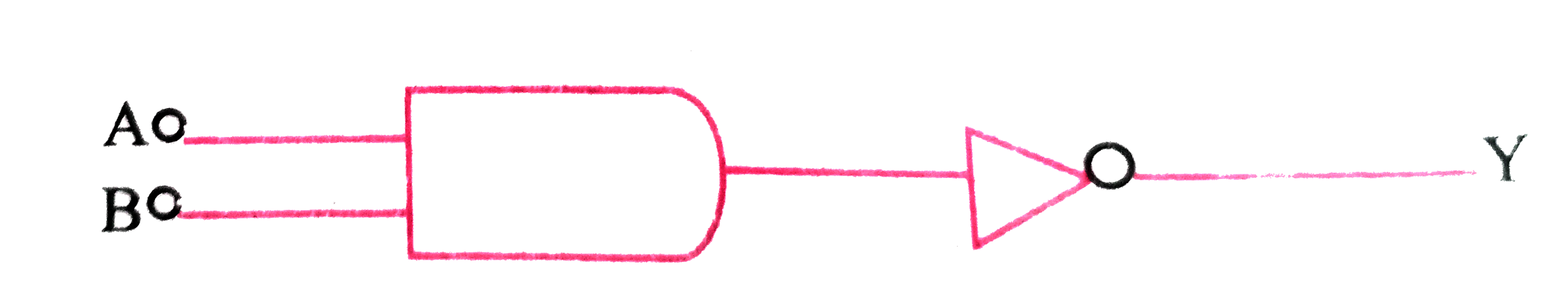
View SolutionWhich of the following statements is correct?
View SolutionMultiple correct postmodern Which of the following statements is correct?
View SolutionWhich of the following statements is / are correct ?
View SolutionChoose the correct option.
Which is following relations is correct?
View Solutionसही उत्तर चुनें-
निम्नलिखित में कौन सही है ?
View Solutionwhich of the following is correct for the following graph?
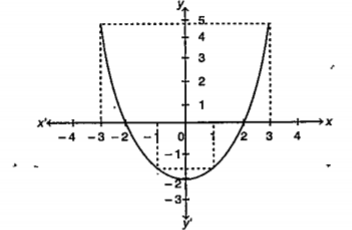
View SolutionWhich of the following statement is//are correct ?
View SolutionWhich of the following statements is/are correct about the following is/are correct about the following reactions ?
View Solution

