CH3−C≡C−HNaNH2−−−−→CH31−−→Li/Liq.NH3−−−−−−−→
The correct Answer is:(##GRBHPJ∃ORGCHEC03E06006_A01##)
Similar Questions
Explain why the chain initiating step in thermal chlorination of CH_(4) is Cl_(2)overset(Delta)rarroverset(.)(C)l and not CH_(4)overset(Delta)rarroverset(.)(C)H_(3)+overset(.)H
View SolutionCH_(3)CH_(2)C-=CHoverset(NaNH_(2)//NH_(3)(liq.))rarr overset(CH_(3)CH_(2)Br)rarroverset(Li,NH_(3)(liq))rarr
View SolutionIn the reaction :
CH3−C≡C−H(i)NaNH2/NH3(I)−−−−−−−−−−−−−→(A)CH3−CH2−CH2−Br−−−−−−−−−−−−−→(B)
the product (B) is :
View SolutionIn the reaction H−C≡CH(1)NaNH2/liq.NH3−−−−−−−−−−−−−→(2)CH3CH2BrX,(i)NaNH2/liq.NH3−−−−−−−−−−−−−→(2)CH3CH2BrY
X and Y are :
View SolutionIn the following chemical reaction H-C-=CHunderset((2)CH_(3)CH_(2)Br)overset(NaNH_(2)//liq.NH_(3))rarr X=underset((2)CH_(3)CH_(2)Br)overset((1)NaNH_(2)//liq.NH_(3))rarrY X and Y are :
View SolutionIn the reaction H-C-=CH underset((ii) CH_(3)CH_(2)Br)overset((I) NaNH_(2)//liq. NH_(3))rarr X underset((ii) CH_(3)CH_(2)Br)overset((I) NaNH_(2)//liq.NH_(3))rarr Y X and Y are .
View SolutionComplete the following reactions: a. H−C(A)≡C−H1mol−−−−→NaNH2(B)CH3Br−−−→(C)

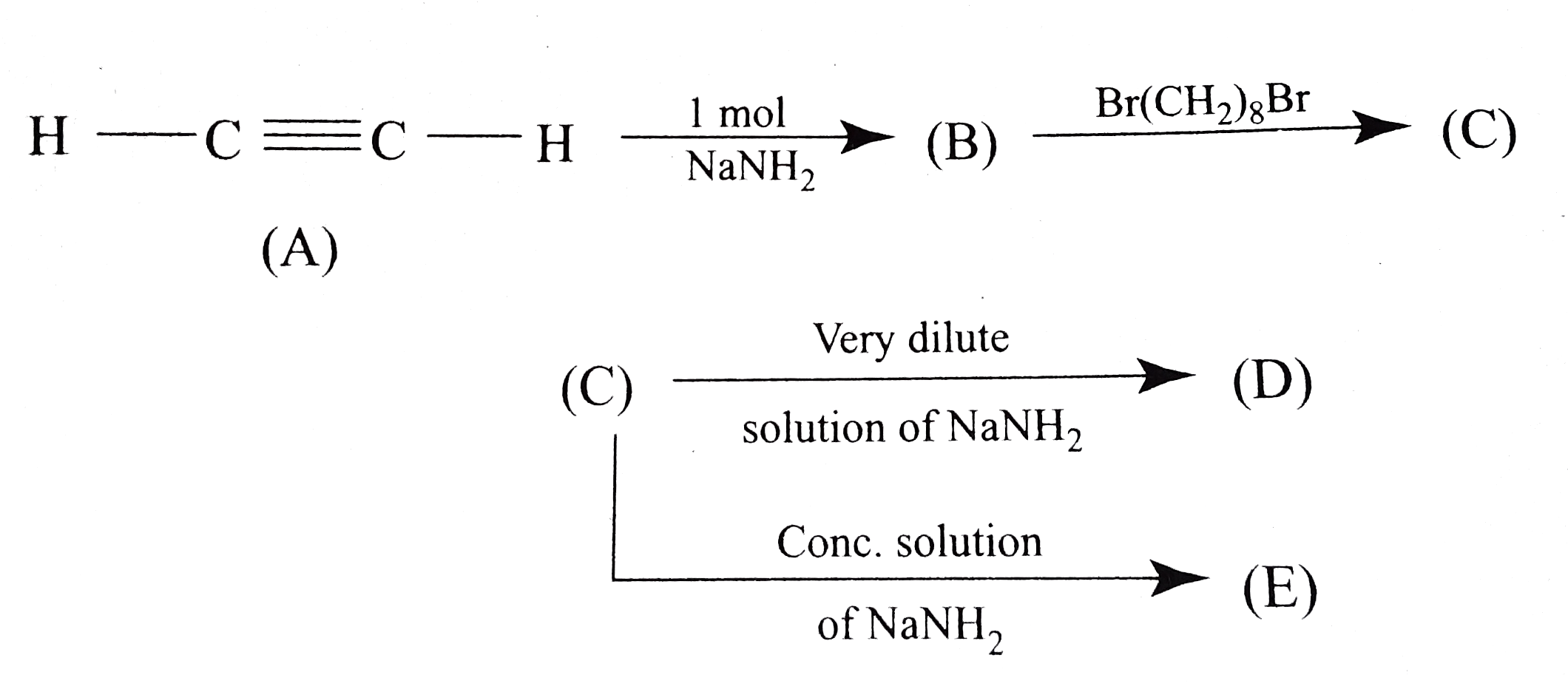
g. H−C≡C(A)−H1mol−−−−→NaNH2(B)Br(CH2)8Br−−−−−−−−→(C)
View SolutionCH3−Cl∣C∣Cl−CH3 to CH3−C≡CNa conversion by using NaNH2/Liq.NH3, Number of moles of NaNH2 required will be
View Solution

