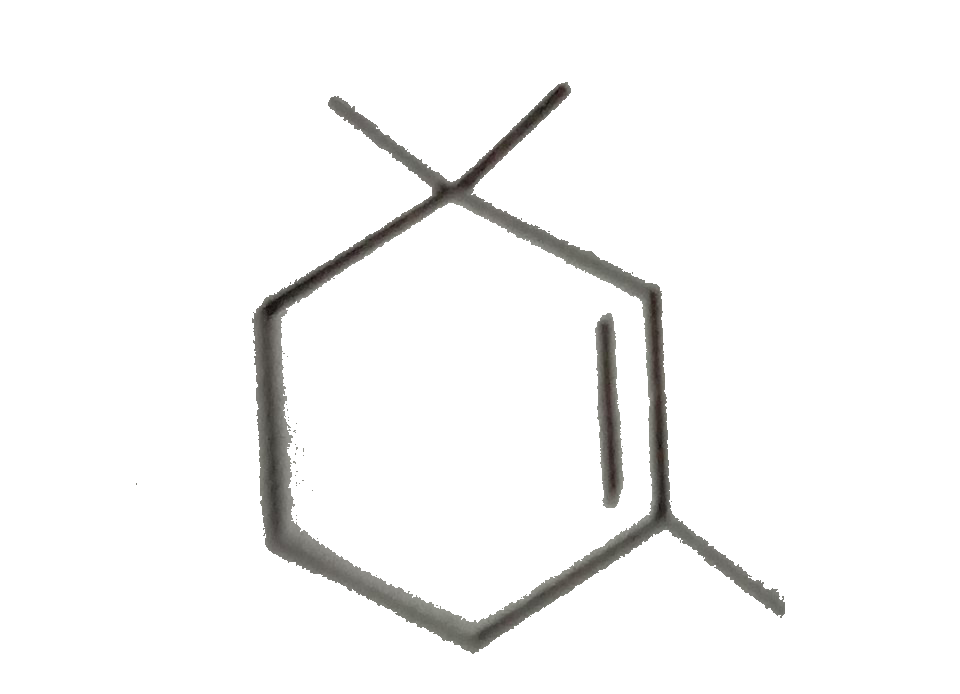
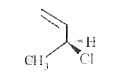Give the chemical name of compound present in tooth enamel. What is the nature of compound?
To answer the question regarding the compound present in tooth enamel and its nature, we can follow these steps:
Step 1: Identify the Compound Present in Tooth Enamel
Tooth enamel is primarily composed of a chemical compound known as calcium phosphate.
Step 2: Write the Chemical Formula
The chemical formula for calcium phosphate is Ca₃(PO₄)₂. This indicates that it contains calcium ions and phosphate ions.
Step 3: Understand the Formation of Calcium Phosphate
Calcium phosphate is formed through a neutralization reaction between phosphoric acid (H₃PO₄), which is a weak acid, and calcium hydroxide (Ca(OH)₂), a strong base. The reaction can be summarized as follows:
- Weak Acid: H₃PO₄
- Strong Base: Ca(OH)₂
- Resulting Salt: Ca₃(PO₄)₂
Step 4: Determine the Nature of Calcium Phosphate
Since calcium phosphate is formed from a weak acid and a strong base, it is classified as a basic salt. This is because the strong base (calcium hydroxide) has a greater influence on the overall nature of the salt compared to the weak acid.
Final Answer
The chemical name of the compound present in tooth enamel is calcium phosphate (Ca₃(PO₄)₂), and the nature of this compound is basic.
---
Tooth enamel is primarily composed of a chemical compound known as calcium phosphate.
The chemical formula for calcium phosphate is Ca₃(PO₄)₂. This indicates that it contains calcium ions and phosphate ions.
Calcium phosphate is formed through a neutralization reaction between phosphoric acid (H₃PO₄), which is a weak acid, and calcium hydroxide (Ca(OH)₂), a strong base. The reaction can be summarized as follows:
- Weak Acid: H₃PO₄
- Strong Base: Ca(OH)₂
- Resulting Salt: Ca₃(PO₄)₂
Since calcium phosphate is formed from a weak acid and a strong base, it is classified as a basic salt. This is because the strong base (calcium hydroxide) has a greater influence on the overall nature of the salt compared to the weak acid.
The chemical name of the compound present in tooth enamel is calcium phosphate (Ca₃(PO₄)₂), and the nature of this compound is basic.
Topper's Solved these Questions
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:ACIDS,BASES AND SALTS (SHORT ANSWER)
Explore 24 Videos
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:ACIDS,BASES AND SALTS (LONG ANSWER)
Explore 22 Videos
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:CHEMICAL REACTION (MULTIPLE CHOICE QUESTION)
Explore 13 Videos
- CHEMICAL REACTIONS AND EQUATIONSBook:OSWAL PUBLICATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:CASE-BASED MCQs
Explore 29 Videos
- METALS AND NON - METALS Book:OSWAL PUBLICATIONChapter:METALS AND NON - METALS Exercise:CASE - BASED MCQs
Explore 29 Videos
Similar Questions
Knowledge Check
What is the name of the compound ?
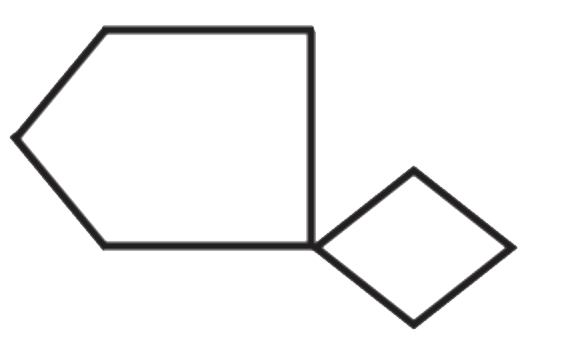 ASpiro [3.4] octaneBSpiro [2.5] octaneCSpiro [3.5] octaneDNone of these
ASpiro [3.4] octaneBSpiro [2.5] octaneCSpiro [3.5] octaneDNone of theseChemical compound present in tooth paste is.
Abasic-Ca3(PO4)2Bacidic-Ca3(PO4)Cbasic-Ca (HCO3)2Dsalt- Ca3P2What is the IUPAC name of compound is:
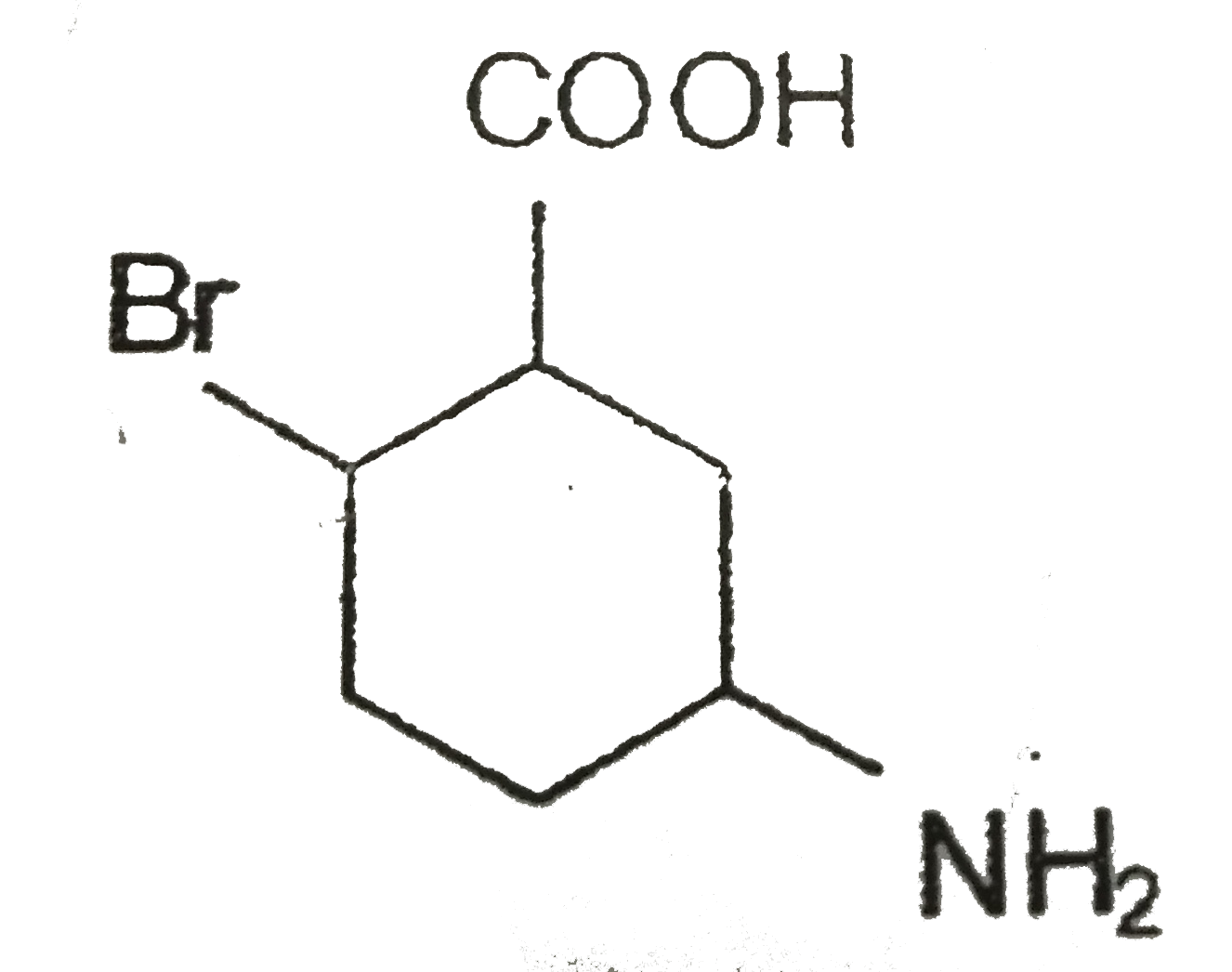 A3-Amino-6-bromocyclohexane-1-carboxylic acidB2-Bromo-5-aminocychexane-1-carboxylic acidC5-Amino-2-bromocyclohexane-1-carboxylic acidD4-Bromo-5-carboxycyclohexanamine
A3-Amino-6-bromocyclohexane-1-carboxylic acidB2-Bromo-5-aminocychexane-1-carboxylic acidC5-Amino-2-bromocyclohexane-1-carboxylic acidD4-Bromo-5-carboxycyclohexanamine