The following questions consist of two statements - Assertion (A) and Reason(R).
Answer the questions selecting the appropriate options given below:
Assertion (A) : C2H4 will undergo addition an reaction with hydrogen
Reason (R): C2H4 is unsaturated compound.
To solve the question, we need to analyze both the Assertion (A) and the Reason (R) provided:
Assertion (A): C2H4 will undergo an addition reaction with hydrogen.
Reason (R): C2H4 is an unsaturated compound.
1. Understanding C2H4:
- The molecular formula C2H4 represents ethene (also known as ethylene).
- Ethene has a double bond between the two carbon atoms, which can be represented as CH2=CH2.
2. Identifying Unsaturation:
- A compound is considered unsaturated if it contains double or triple bonds. Since C2H4 has a double bond, it is classified as an unsaturated compound.
3. Addition Reactions:
- Addition reactions typically occur with unsaturated compounds. In these reactions, atoms or groups of atoms are added to the compound, usually across a double or triple bond.
- When hydrogen (H2) is added to an unsaturated compound like ethene, it can convert the double bond into a single bond, resulting in a saturated compound.
4. Reaction of C2H4 with Hydrogen:
- When C2H4 reacts with hydrogen in the presence of a catalyst (such as nickel), the reaction can be represented as:
\(
\text{C2H4} + \text{H2} \xrightarrow{\text{Ni}} \text{C2H6}
\)
- Here, C2H6 is ethane, which is a saturated compound.
5. Conclusion:
- Since C2H4 (ethene) is indeed an unsaturated compound and it undergoes an addition reaction with hydrogen, both the assertion and the reason are true.
- Furthermore, the reason correctly explains why the assertion is true.
Final Answer:
Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of Assertion (A). Therefore, the correct option is Option 1.
---
Reason (R): C2H4 is an unsaturated compound.
- The molecular formula C2H4 represents ethene (also known as ethylene).
- Ethene has a double bond between the two carbon atoms, which can be represented as CH2=CH2.
- A compound is considered unsaturated if it contains double or triple bonds. Since C2H4 has a double bond, it is classified as an unsaturated compound.
- Addition reactions typically occur with unsaturated compounds. In these reactions, atoms or groups of atoms are added to the compound, usually across a double or triple bond.
- When hydrogen (H2) is added to an unsaturated compound like ethene, it can convert the double bond into a single bond, resulting in a saturated compound.
- When C2H4 reacts with hydrogen in the presence of a catalyst (such as nickel), the reaction can be represented as:
\(
\text{C2H4} + \text{H2} \xrightarrow{\text{Ni}} \text{C2H6}
\)
- Here, C2H6 is ethane, which is a saturated compound.
- Since C2H4 (ethene) is indeed an unsaturated compound and it undergoes an addition reaction with hydrogen, both the assertion and the reason are true.
- Furthermore, the reason correctly explains why the assertion is true.
Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of Assertion (A). Therefore, the correct option is Option 1.
Topper's Solved these Questions
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (VERY SHORT ANSWER)
9 Videos
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (SHORT ANSWER)
22 Videos
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - CARBON COMPOUNDS (LONG ANSWER)
6 Videos
- CHEMICAL REACTIONS AND EQUATIONSBOOK - OSWAL PUBLICATIONCHAPTER - CHEMICAL REACTIONS AND EQUATIONSEXERCISE - CASE-BASED MCQs
29 Videos
- METALS AND NON - METALS BOOK - OSWAL PUBLICATIONCHAPTER - METALS AND NON - METALS EXERCISE - CASE - BASED MCQs
29 Videos
Similar Questions
Which element was named as Eka-aluminium earlier?
View SolutionTwo elements 'A' and 'B' have atomic numbers 11 and 19 respectively. State their position in the Modern Periodic Table.
Which element has bigger atomic radius.
What is the nature of their oxides.
View SolutionWhat is a homologous series ? Explain with an example.
View SolutionMarble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is
View SolutionRead the following ans answer any four metals A,B,C and D added following solutions one. The results obtained have tabulated as follows :
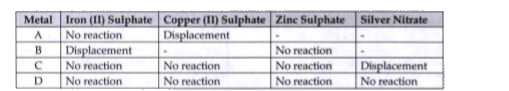
Choose the most reactive metal:
View SolutionThe questions given below consist of Assertion (A) and Reason (R ) . Use the following key to select the correct answer.
assertion : Cis[CoCl2(en)2]2+ is optically active in nature .
Reason : It has no plane of symmetry .
View SolutionThe questions given below consist of Assertion (A) and Reason (R ) . Use the following key to select the correct answer.
Assertion : The ligand N−3 is named as nitrido.
Reason : It is derived from NH3.
View SolutionThe questions given below consist of Assertion (A) and Reason (R ) . Use the following key to select the correct answer. Assertion : The ligands nitrio-N and nitrio-O are ambident in nature . Reason : The ligands give linkage isomers.
View SolutionThe questions given below consist of Assertion (A) and Reason (R ) . Use the following key to select the correct answer.
Assertion : Ni(dmg)2 is a square planar complex.
Reason : Chelation effect is present in Ni(dmg)2.
View SolutionThe questions given below consist of Assertion (A) and Reason (R ) . Use the following key to select the correct answer.
Assertion : In Zeise's salt, co-ordianation number of Pt is five.
Reason : Ethene is a bidentate lignad .
View SolutionIn the question given below, there are two statements marked as Assertion (A) and Reason (R) Read the statements and choose the correct option:
Assertion (A): Democracy is a legitimate government.
Reason (R): Regular, free and fair elections are the spirit of democracy. Options:
View SolutionIn the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): Combustion of all organic compounds is an exothermic reaction.
Reason (R) : The enthalpies of all elements in their standard state are zero.
View SolutionAssertion (A) : Unsatured organic com pounds undergo substitution reactions. Reason (R) : Unsaturated organic compounds contain=or≡bonds.
View SolutionThe questions given below consist of Assertion (A) and Reason (R ) . Use the following key to select the correct answer. Assertion : Ni("dmg")_2 is a square planar complex. Reason : Chelation effect is present in Ni("dmg")_2 .
View SolutionThe questions given below consist of Assertion (A) and Reason (R ) . Use the following key to select the correct answer. Assertion : In Zeise's salt, co-ordianation number of Pt is five. Reason : Ethene is a bidentate lignad .
View Solution
