According to which periodic law, every eight element repeat its property?
Step-by-Step Solution:
1. Identify the Question: The question asks about a periodic law that states every eighth element repeats its properties.
2. Recall the Relevant Law: The law that describes this phenomenon is known as Newland's Law of Octaves.
3. Understanding Newland's Law: Newland's Law of Octaves states that when elements are arranged in increasing order of their atomic mass, the properties of every eighth element are similar to the properties of the first element in the sequence.
4. Example for Clarity: To illustrate this, consider the first few elements:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Sodium (Na)
- Magnesium (Mg)
If we take Lithium (Li) as the starting element, the eighth element from Lithium is Sodium (Na). According to Newland's Law, Lithium and Sodium will have similar properties.
5. Conclusion: Therefore, the answer to the question is that the periodic law according to which every eighth element repeats its properties is Newland's Law of Octaves.
---
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Sodium (Na)
- Magnesium (Mg)
Topper's Solved these Questions
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:PERIODIC CLASSIFICATION OF ELEMENTS (SHORT ANSWER)
Explore 22 Videos
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:PERIODIC CLASSIFICATION OF ELEMENTS (LONG ANSWER)
Explore 30 Videos
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:CARBON COMPOUNDS (MULTIPLE CHOICE QUESTIONS)
Explore 14 Videos
- CHEMICAL REACTIONS AND EQUATIONSBook:OSWAL PUBLICATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:CASE-BASED MCQs
Explore 29 Videos
- METALS AND NON - METALS Book:OSWAL PUBLICATIONChapter:METALS AND NON - METALS Exercise:CASE - BASED MCQs
Explore 29 Videos
Similar Questions
Two elements 'A' and 'B' have atomic numbers 11 and 19 respectively. State their position in the Modern Periodic Table.
Which element has bigger atomic radius.
What is the nature of their oxides.
View SolutionWhich important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
View SolutionAssertion: Chemical bonds in organic compounds are covalent in nature.
Reason: Covalent bond is formed by the sharing of electrons in the bonding atoms.
View SolutionMarble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is
View SolutionRead the following ans answer any four metals A,B,C and D added following solutions one. The results obtained have tabulated as follows :
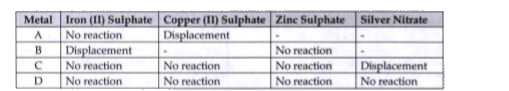
Choose the most reactive metal:
View SolutionAccording to Mendeleef's periodic law, the properties of elements are periodic function of
View SolutionAccording to modern periodic law the elements with similar chemical and physical properties repeat at regular intervals when the elements are arranged in order of :
View Solutionआधुनिक आवर्त नियम के अनुसार , तत्त्वों के गुण आवर्तफलन होते है उनके
View SolutionAccording to Mendeleef's periodic law, the properties of elements are periodic function of
View SolutionAccording to modern periodic law, the physical and chemical properties of elements are the periodic functions of their ?
View SolutionAccording to modern periodic law the elements with similar chemical and physical properties repeat at regular intervals when the elements are arranged in order of :
View SolutionAccording to Mendeleev's periodic law, the properties of elements are a periodic function of their atomic numbers.
View SolutionAccording to Mendeleev's periodic table , the physical and chemical properties of elements are periodic function of their __________
View SolutionAccording to modern periodic law, the physical and chemical properties of the elements are periodic function of their:
View SolutionAccording to modern periodic law , the chemical properties of elements are the periodic functions of there :
View SolutionAccording to Mendeleev's periodic law the properties of elements were the periodic function of :
View Solution