Priya took an element X which is a yellow solid at room temperature and shows catenation. X also forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals.
Write the electronic configuration of X.
2,8,6
टॉपर्स ने हल किए ये सवाल
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:PERIODIC CLASSIFICATION OF ELEMENTS (MULTIPLE CHOICE QUESTIONS)
Explore 13 Videos
- DIKSHA QUESTIONS Book:OSWAL PUBLICATIONChapter:DIKSHA QUESTIONS Exercise:PERIODIC CLASSIFICATION OF ELEMENTS (SHORT ANSWER)
Explore 22 Videos
- CHEMICAL REACTIONS AND EQUATIONSBook:OSWAL PUBLICATIONChapter:CHEMICAL REACTIONS AND EQUATIONSExercise:CASE-BASED MCQs
Explore 29 Videos
- METALS AND NON - METALS Book:OSWAL PUBLICATIONChapter:METALS AND NON - METALS Exercise:CASE - BASED MCQs
Explore 29 Videos
समान प्रश्न
The electronic configuration of an element M is 2,8,4. In modern periodic table, the element M is placed in
View SolutionTwo elements 'A' and 'B' have atomic numbers 11 and 19 respectively. State their position in the Modern Periodic Table.
Which element has bigger atomic radius.
What is the nature of their oxides.
View SolutionMarble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is
View SolutionRead the following ans answer any four metals A,B,C and D added following solutions one. The results obtained have tabulated as follows :
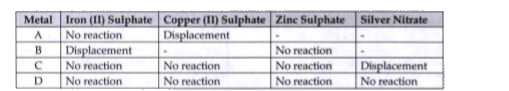
Choose the most reactive metal:
View SolutionAll the group 2 elements burn in O_(2) to form oxides MO. These oxides can also be obtained by the thermal decomposition of (i) carbonates , (ii) nitrates (iii) sulphates, (iv) hydroxides
View SolutionAn element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants (a) Identify the element X (b) Write the electronic configuration of X (c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals ? (d) What would be the nature (acidic/basic) of oxides formed ? (e) Locate the position of the element in the modern table.
View SolutionAn element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants. (a) Identify the element X. (b) Write the electronic configuration of X. (c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals. (d) What would be the nature (acidic/basic) of oxides formed? (e) Locate the position of the element in the Modern Periodic Table.
View Solutionफेरस सल्फेट के अपघटन से निम्न में से किस तत्व का ऑक्साइड निर्मित होता है
View SolutionAn element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants
(a) Identify the element X
(b) Write the electronic configuration of X
(c) Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals ?
(d) What would be the nature (acidic/basic) of oxides formed ?
(e) Locate the position of the element in the modern periodic table.
View Solutionनिम्न में से कौन-सा तत्व जलकर ऑक्साइड बनाता है, जो कमरे के ताप पर गैस है?
View SolutionThe gases release during the decomposition of Ferrous Sulphate will show which change
View Solutionएक तत्व X जो कक्ष ताप पर पीला ठोस हैं शृंखलन तथा अपररूपता दर्शाता हैं। X दो प्रकार के ऑक्साइड बनाता हैं जो कि फैरस सल्फेट क्रिस्टल के तापीय विघटन के दौरान भी बनते हैं तथा प्रमुख वायु प्रदूषक हैं।
(a) तत्व X को पहचानिए।
X का इलेक्ट्रॉनिक विन्यास लिखिए।
(c ) फैरस सल्फेट क्रिस्टल के तापीय अपघटन की संतुलित रासायनिक समीकरण लिखिए।
(d) बनने वाले ऑक्साइड की प्रकृति (अम्लीय / क्षारकीय ) क्या होगी ?
(e ) आधुनिक आवर्त सारणी में तत्व की स्थिति बताइये।
View SolutionAn element X has the electronic configuraiton 2,8,8,2. ON the basis of electronic configuration,
Does this element form acidic or basic oxide?
View SolutionPriya took an element X which is a yellow solid at room temperature and shows catenation. X also forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals.
Identify the element X.
View SolutionPriya took an element X which is a yellow solid at room temperature and shows catenation. X also forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals.
Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals.
View Solution