The valency of an element with atomic number 8 is :
7
6
2
1
The correct Answer is:C
To determine the valency of the element with atomic number 8, we can follow these steps:
Step 1: Identify the Element
The atomic number 8 corresponds to the element Oxygen (O).
Step 2: Determine the Electronic Configuration
For an element with atomic number 8, the electronic configuration can be determined as follows:
- The first shell (K shell) can hold a maximum of 2 electrons.
- The second shell (L shell) can hold a maximum of 8 electrons.
Thus, the electronic configuration of Oxygen is:
- K shell: 2 electrons
- L shell: 6 electrons
So, the complete electronic configuration is 1s² 2s² 2p⁴, which shows that Oxygen has 6 electrons in its outermost shell (valence shell).
Step 3: Determine the Valency
Valency is defined as the number of electrons an atom can lose, gain, or share to achieve a stable electronic configuration (octet).
In the case of Oxygen:
- It has 6 electrons in its valence shell.
- To achieve a stable octet (8 electrons), it needs to gain 2 more electrons.
Step 4: Conclusion
Since Oxygen needs to gain 2 electrons to complete its octet, its valency is 2.
Thus, the valency of the element with atomic number 8 (Oxygen) is 2.
The atomic number 8 corresponds to the element Oxygen (O).
For an element with atomic number 8, the electronic configuration can be determined as follows:
- The first shell (K shell) can hold a maximum of 2 electrons.
- The second shell (L shell) can hold a maximum of 8 electrons.
- K shell: 2 electrons
- L shell: 6 electrons
Valency is defined as the number of electrons an atom can lose, gain, or share to achieve a stable electronic configuration (octet).
- It has 6 electrons in its valence shell.
- To achieve a stable octet (8 electrons), it needs to gain 2 more electrons.
Since Oxygen needs to gain 2 electrons to complete its octet, its valency is 2.
Topper's Solved these Questions
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (LONG ANSWER)
30 Videos
- CHEMICAL REACTIONS AND EQUATIONSBOOK - OSWAL PUBLICATIONCHAPTER - CHEMICAL REACTIONS AND EQUATIONSEXERCISE - CASE-BASED MCQs
29 Videos
- METALS AND NON - METALS BOOK - OSWAL PUBLICATIONCHAPTER - METALS AND NON - METALS EXERCISE - CASE - BASED MCQs
29 Videos
Similar Questions
Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
View SolutionMarble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is
View SolutionRead the following ans answer any four metals A,B,C and D added following solutions one. The results obtained have tabulated as follows :
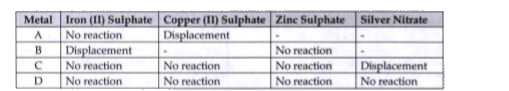
Choose the most reactive metal:
View SolutionThe atomic number of an element variable valency.
View SolutionThe atomic number of an element X is 8 and that of element Y is 4. Both these elements can exhibit a valency of :
View Solutionपरमाणु संख्या 6,7 और 8 वाले तत्वों की संयोजकता एवं उनके इलेक्ट्रॉनिक विन्यास लिखें । इनमे किस प्रकार की संयोजकता हैं और क्यों ?
View SolutionWhat is the number of valence electrons in the atom of an element A having atomic number 17 ? Name the valence shell of this atom.
View SolutionThe sum of number of valence electrons and valency of an element with atomic number 15 is_____.
View Solution9 परमाण्विक संख्या वाले तत्व की संयोजकता है
View SolutionWhat is the valency of elements with atomic number 8, 14, 17 and 20?
View SolutionThe atomic number of element X is 8, while the atomic number of element Y is 4 . Hence. The valency of these elements is ...... .
View Solutionपरमाणु की संयोजकता क्या होगी, यदि उसका परमाण क्रमांक 8 है?
View Solutionपरमाणु की संयोजकता क्या होगी, यदि उसका परमाण क्रमांक 8 है?
View SolutionThe sum of number of valence electrons and valency of an element with atomic number 15 is_____.
View SolutionValency expresses… total electrons in valence shell of an atom. atomicity of an element . oxidation number of an element. combinig capacity of an element.
View SolutionWhat is the number of valence electrons in the atom of an element A having atomic number 20? Name the valence shell of this atom.
View Solution
