Read the given passage and answer the questions.
Suhana wanted her house to be white washed, She bought 10 kg of quicklime from the market and dissolved it in 30 L of water. On adding lime to water, she observed that the water started boiling even when it was not being heated.
Which of the following statements is correct about the above reaction based on your observations?
(i) It is an endothermic reaction.
(ii) It is an exothermic reaction
(iii) The pH of the resulting solution will be more than seven.
(iv) The pH of the resulting solution will be less than seven.
(i) and (ii)
(ii) and (iii)
(i) and (iv)
(iii) and (iv)
The correct Answer is:B
To solve the question, we will analyze the reaction that occurs when quicklime (calcium oxide, CaO) is added to water. The steps are as follows:
Step 1: Identify the Reaction
When quicklime (CaO) is added to water, it reacts to form slaked lime (calcium hydroxide, Ca(OH)₂). The reaction can be represented as:
CaO (s)+H2O (l)→Ca(OH)2(aq)
Step 2: Determine the Nature of the Reaction
The passage states that the water started boiling even when it was not being heated. This indicates that heat is being released during the reaction.
- Exothermic Reaction: In an exothermic reaction, heat is released into the surroundings, causing an increase in temperature. Since the water boiled, this suggests that the reaction is exothermic.
Step 3: Analyze the pH of the Resulting Solution
The resulting solution after the reaction is calcium hydroxide, which is a base.
- pH of Basic Solutions: The pH of a basic solution is greater than 7. Therefore, the pH of the resulting solution will be more than 7.
Step 4: Evaluate the Given Statements
Now, we will evaluate the provided statements based on our analysis:
1. (i) It is an endothermic reaction. - Incorrect (The reaction is exothermic, not endothermic.)
2. (ii) It is an exothermic reaction. - Correct (The reaction releases heat.)
3. (iii) The pH of the resulting solution will be more than seven. - Correct (The solution is basic.)
4. (iv) The pH of the resulting solution will be less than seven. - Incorrect (The solution is not acidic.)
Conclusion
The correct statements based on the observations are (ii) and (iii).
Final Answer
- The correct statements are:
- (ii) It is an exothermic reaction.
- (iii) The pH of the resulting solution will be more than seven.
---
When quicklime (CaO) is added to water, it reacts to form slaked lime (calcium hydroxide, Ca(OH)₂). The reaction can be represented as:
CaO (s)+H2O (l)→Ca(OH)2(aq)
The passage states that the water started boiling even when it was not being heated. This indicates that heat is being released during the reaction.
The resulting solution after the reaction is calcium hydroxide, which is a base.
Now, we will evaluate the provided statements based on our analysis:
2. (ii) It is an exothermic reaction. - Correct (The reaction releases heat.)
3. (iii) The pH of the resulting solution will be more than seven. - Correct (The solution is basic.)
4. (iv) The pH of the resulting solution will be less than seven. - Incorrect (The solution is not acidic.)
The correct statements based on the observations are (ii) and (iii).
- The correct statements are:
- (ii) It is an exothermic reaction.
- (iii) The pH of the resulting solution will be more than seven.
Topper's Solved these Questions
- ACIDS, BASES AND SALTSBOOK - OSWAL PUBLICATIONCHAPTER - ACIDS, BASES AND SALTSEXERCISE - Self Assessment - 1 (I. Objective Type Questions) A. Multiple Choice Questions
3 Videos
- ACIDS, BASES AND SALTSBOOK - OSWAL PUBLICATIONCHAPTER - ACIDS, BASES AND SALTSEXERCISE - Self Assessment - 1 (I. Objective Type Questions) B. Passage / Table Based Questions
4 Videos
- ACIDS, BASES AND SALTSBOOK - OSWAL PUBLICATIONCHAPTER - ACIDS, BASES AND SALTSEXERCISE - ASSERTION AND REASON BASED MCQs
8 Videos
- CARBON AND ITS COMPOUNDSBOOK - OSWAL PUBLICATIONCHAPTER - CARBON AND ITS COMPOUNDSEXERCISE - CREATING BASED QUESTIONS
10 Videos
Similar Questions
Which of the following statements about acids is correct ?
View SolutionStudy the given table and answer the following questions. It shows the pH value of the plaque (which collects around teeth) surrounding the teeth of a child over three hours.
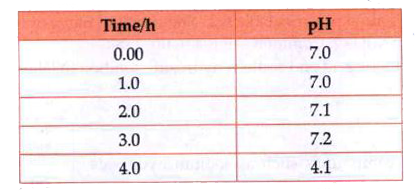
The three constituents of plaque are :
View SolutionAssertion (A) : After white washing the walls, a shiny white finish on walls is obtained after two to three days.
Reason (R ) : Calcium Oxide reacts with Carbon dioxide to form Calcium Hydrogen Carbonate which gives shiny white finish.
View SolutionThe solid element A exhibits the property of catenation. It is also present in the form of a gas B in the air which is utilised by plants in photosynthesis. An allotrope C of this element is used in glass cutters.
What is element A?
View SolutionObserve the following reaction: Which statement is not correct about the above observation?
View SolutionWhen ethanol dissolves in water, which of the following is observed?
View SolutionA house wife wanted her house to be white washed. She bought 10 kg of quick lime from the market and dissolved in 30 litres of water. She noticed that water started boiling even when it was not being heated. Give reason for her observation. Write the corresponding equation and name the product formed.
View SolutionThe process of preparing slaked lime by adding water to quicklime is this type of chemical reaction.
View SolutionThe reaction that takes place when quicklime is added to water is ………….
View SolutionYou should not put your hands in the vessel while preparing quicklime from slaked lime by adding water to it because ……………
View Solution
Observe the above diagram and answer the following questions :
Why the lime water is used in this experiment ?
View SolutionWrite your observations for the chemical reactions and name the product formed :
When water is added to quick lime?
View SolutionWhat do you observe when Water is added to quicklime.
View SolutionObserve the table: Which gas turns lime water to milky white?
View SolutionHave you observed any change when lime water is added to turmeric ?
View SolutionAnu wants to boil water quickly in a test tube. On observing the different zones of the flame, she is not able to decide which zone of the flame will be best for boiling water quickly. Help her in this activity.
View Solution
