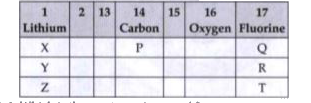
Name the element P placed below Carbon in group 14:
Aluminium
Silicon
Phosphorus
Sulphur
The correct Answer is:B
Silicon is the element placed below carbon in group. 14.
Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTS Book:OSWAL PUBLICATIONChapter:PERIODIC CLASSIFICATION OF ELEMENTS Exercise:SELF - ASSESSMENT - 1 ( OBJECTIVE TYPE QUESTIONS)
Explore 13 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS Book:OSWAL PUBLICATIONChapter:PERIODIC CLASSIFICATION OF ELEMENTS Exercise:SELF - ASSESSMENT - 2 ( OBJECTIVE TYPE QUESTIONS)
Explore 15 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS Book:OSWAL PUBLICATIONChapter:PERIODIC CLASSIFICATION OF ELEMENTS Exercise:ASSERTION AND REASON BASED MCQs
Explore 9 Videos
- OLYMPIAD 2019 - 20Book:OSWAL PUBLICATIONChapter:OLYMPIAD 2019 - 20Exercise:CHEMISTRY QUESTIONS (Is Matter Around us Pure)
Explore 1 Video
Similar Questions
From the part of the periodic table given, answer the following questions.
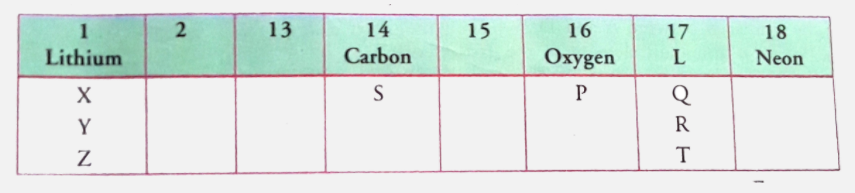
(a) Which is the most reactive metal ? (b) Name the family of L, Q, R, T.
(c) Name one element of group 2 and 15, (d) Name one member of group 18 other than neon. (e) Give the name of the element S placed below carbon in group 14.
View SolutionName the first element of Group 3.
View Solution
Knowledge Check
The electronic configuration of four different elements is given below. Identify the group 14 elements among these.
A[He]2s1B[Ne]3s2C[Ne]3s23p2D[Ne]3s23p5Atomic number of Ag is 47. in the same group, the atomic number of elements placed above and below Ag will be:
A37,67B29,79C39,69D18,28
Name the first element of group 16
View Solutionwhat are the three group into which all the elements can be divided ? Name two elements belonging to each group .
View SolutionName two elemental forms of carbon.
View SolutionIf an element X is placed in group 14, what will be the formula and the nature of bonding of its chloride ?
View SolutionIf an element X is placed in group 14, what will be the formula and the nature of bonding of its chloride ?
View SolutionName the radioactive element of group 1
View Solution