Hydrogen bond energy is equal to :
A
3-7 cal
B
30-70 cal
C
3-10 kcal
D
30-70 kcal
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
Hydrogen bonding is
Aa weak type of dipole-dipole interactionBa strong type of dipole-dipole interactionCa strong type of dipole-induced dipole interactionDAll of the aboveHydrogen bonding is maximum in
AEthanolBDiethyl etherCEthyl chlorideDTriethyl amineA : Bond energy is equal to enthalpy of formation with negative sign.
R: Bond energy is energy required to dissociate 1 mole single bond.AIf both Assertion & Reason are true and the reason is the correct explanation of the assertion , then mark (1)BIf both Assertion & Reason are true and the reason is not the correct explanation of the assertion, then mark (2)CIf Assertion is true statement but Reason is false, then mark (3)DIf both Assertion is true statement but Reason is false, then mark (3)
Hydrogen Bonding
View SolutionHydrogen bond energy is around ............ kJ mol−1
View SolutionA : Bond energy is equal to enthalpy of formation with negative sign.
R: Bond energy is energy required to dissociate 1 mole single bond.
View SolutionThe bond dissociation energy depends upon the nature of the bond and nature of the molecule. If any molecule more than 1 bonds of similar nature are present then the bond energy reported is the average bond energy.
If enthalpy of hydrogenation of C6H6(l)intoC6H12(l) is −205kJ and resonance energy of C6H6(l) is −152kJ/mol then enthalpy of hydrogenation of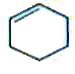 is ? Answer ΔHvap of C6H6(l),C6H10(l),C6H12(l) all are equal:
is ? Answer ΔHvap of C6H6(l),C6H10(l),C6H12(l) all are equal:
View SolutionHydrogen bonding is not shown by
View Solution

