Compounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an Activity to prove it.
Two nails are fitted on a cork and are kept it in a 100 mL beaker. The nails are then connected to the two terminals of a 6-volt battery through a bulb and a switch. Some dilute HCl is poured in the beaker and the current is switched on. The same experiment is then performed with glucose solution and alcohol solution.
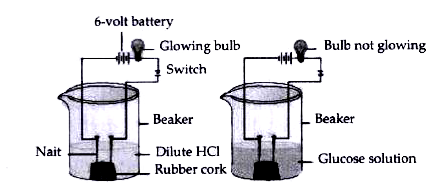
(i) Observations : It will be observed that the bulb glows in the HCl solution and does not glow in the glucose solution.
(ii) Result : HCl dissociates into H+ and Cl− ions. These ions conduct electricity in the solution resulting in the glowing of the bulb. On the other hand, the glucose solution does not dissociate into ions. Therefore, it does not conduct electricity.
(iii) Conclusion : From this activity, it can be concluded that all acids contain hydrogen but not all compounds containing hydrogen are acids. That is why, though alcohols and glucose contain hydrogen, they are not categorised as acids.
Topper's Solved these Questions
- ACIDS, BASES AND SALTSBOOK - OSWAL PUBLICATIONCHAPTER - ACIDS, BASES AND SALTSEXERCISE - NCERT EXEMPLAR (Multiple Choice Type Questions )
53 Videos
- ACIDS, BASES AND SALTSBOOK - OSWAL PUBLICATIONCHAPTER - ACIDS, BASES AND SALTSEXERCISE - Board Corner (Single Answer Type Questions)
18 Videos
- ACIDS, BASES AND SALTSBOOK - OSWAL PUBLICATIONCHAPTER - ACIDS, BASES AND SALTSEXERCISE - NCERT Corner (Exercise Questions )
4 Videos
- CARBON AND ITS COMPOUNDSBOOK - OSWAL PUBLICATIONCHAPTER - CARBON AND ITS COMPOUNDSEXERCISE - CREATING BASED QUESTIONS
10 Videos
Similar Questions
What happens when a solution of an acid is mixed with a solution of a base in a test tube ?
(i) The temperature of the solution increases
(ii) The temperature of the solution decreases
(iii) The temperature of the solution remains the same
(iv) Salt formation takes place
View SolutionA compound 'X' of sodium is used as an antacid and it decomposes on strong heating.
Name the compound 'X' and give its chemical Formula.
View SolutionA solution turns red litmus blue, its pH is likely to be
View SolutionThe solid element A exhibits the property of catenation. It is also present in the form of a gas B in the air which is utilised by plants in photosynthesis. An allotrope C of this element is used in glass cutters.
What is element A?
View SolutionCompounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
View Solutionऐल्कोहॉल एवं ग्लूकोज जैसे यौगिकों में भी हाइड्रोजन होते हैं लेकिन इनका वर्गीकरण अम्ल की तरह नहीं होता है। एक क्रियाकलाप द्वारा इसे साबित कीजिए।
View Solutionऐल्कोहॉल एवं ग्लूकोज जैसे यौगिकों में भी हाइड्रोजन होते है लेकिन इनका वर्गीकरण अम्ल की तरह नहीं होता है । एक क्रियाकलाप द्वारा इसे साबित कीजिए ।
View Solutionऐल्कोहॉल एवं ग्लूकोज जैसे यौगिकों में भी हाइड्रोजन होते है लेकिन इनका वर्गीकरण अम्ल की तरह नहीं होता है । एक क्रियाकलाप द्वारा इसे साबित कीजिए ।
View SolutionCompounds such as alcohols and glucose contain hydrogen but are not categorized as acids. Describe an activity to prove it
View SolutionCompounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an Activity to prove it.
View Solutionऐल्कोहॉल एवं ग्लूकोस जैसे यौगिकों में भी हाइड्रोजन होते हैं लेकिन इसका वर्गीकरण अम्ल की तरह नहीं होता है। एक क्रियाकलाप द्वारा इसे साबित कीजिए |
View SolutionCompounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an Activity to prove it.
View SolutionCompounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an Activity to prove it.
View SolutionCompounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
View SolutionCompounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
View SolutionCompounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an Activity to prove it.
View Solution
