In the given reaction, H2SO4 act as _____
2CH3CH2OHEthanolHot Conc.−−−−−→H2SO42CHEthene=CH2+H2O
Reducing agent
Oxidising agent
Dehydrating agent
Catalyst
To determine how H2SO4 acts in the given reaction, we can analyze the reaction step by step.
Step 1: Identify the Reactants and Products
The reactants in this reaction are ethanol (2CH3CH2OH) and concentrated sulfuric acid (H2SO4). The products formed are ethene (2CH=CH2) and water (H2O).
Hint: Look at the chemical equation to identify what substances are being transformed.
Step 2: Understand the Reaction Conditions
The reaction is carried out under hot and concentrated conditions. This indicates that the reaction is likely to involve significant changes in the structure of the reactants.
Hint: Consider how temperature and concentration can affect chemical reactions, especially in terms of energy and reactivity.
Step 3: Analyze the Reaction Mechanism
In this reaction, ethanol undergoes a process where a water molecule is removed. This process is known as dehydration. The sulfuric acid acts to facilitate this removal of water from ethanol.
Hint: Think about what dehydration means in a chemical context and how it relates to the removal of water.
Step 4: Identify the Role of H2SO4
Since H2SO4 is helping to remove a water molecule from ethanol, it is functioning as a dehydrating agent. A dehydrating agent is a substance that promotes the removal of water from another compound.
Hint: Recall the definition of a dehydrating agent and how it applies to the context of this reaction.
Conclusion
In conclusion, H2SO4 acts as a dehydrating agent in the reaction, facilitating the conversion of ethanol to ethene by removing water.
Final Answer: H2SO4 acts as a dehydrating agent in the given reaction.
The reactants in this reaction are ethanol (2CH3CH2OH) and concentrated sulfuric acid (H2SO4). The products formed are ethene (2CH=CH2) and water (H2O).
The reaction is carried out under hot and concentrated conditions. This indicates that the reaction is likely to involve significant changes in the structure of the reactants.
In this reaction, ethanol undergoes a process where a water molecule is removed. This process is known as dehydration. The sulfuric acid acts to facilitate this removal of water from ethanol.
Since H2SO4 is helping to remove a water molecule from ethanol, it is functioning as a dehydrating agent. A dehydrating agent is a substance that promotes the removal of water from another compound.
In conclusion, H2SO4 acts as a dehydrating agent in the reaction, facilitating the conversion of ethanol to ethene by removing water.
Topper's Solved these Questions
- NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION Book:OSWAL PUBLICATIONChapter:NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION Exercise:CARBON AND ITS COMPOUNDS (SELF ASSESSMENT) (OBJECTIVE TYPE QUESTIONS) (B. Assertion and Reason Type Questions )
Explore 2 Videos
- NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION Book:OSWAL PUBLICATIONChapter:NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION Exercise:CARBON AND ITS COMPOUNDS (SELF ASSESSMENT) (C. Very Short Answer Type Questions)
Explore 3 Videos
- NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION Book:OSWAL PUBLICATIONChapter:NEW CHAPTERS AND QUESTIONS BASED ON LATEST TYPOLOGIES INTRODUCED BY CBSE FOR 2021-22 EXAMINATION Exercise:METALS AND NON- METALS (VISUAL CASE - BASED QUESTIONS)
Explore 15 Videos
- METALS AND NON-METALSBook:OSWAL PUBLICATIONChapter:METALS AND NON-METALSExercise:Board corner(Long answer type questions)
Explore 20 Videos
- NTSE 2019 - 20 Book:OSWAL PUBLICATIONChapter:NTSE 2019 - 20 Exercise:(CHEMISTRY) ATOMS AND MOLECULES (STAGE - 2)
Explore 1 Video
Similar Questions
Assertion (A): In esterification, carboxylic acid and alcohol reacts in the presence of acid to give ester.
Reason (R) : Esterification is the reverse of saponification.
View SolutionName the process of converting vegetable oil to vegetable ghee.
View SolutionDuring an experiment, Rohan took the samples of four metals A, B, C and D and added following solution one by one. The results obtained have been tabulated as below:
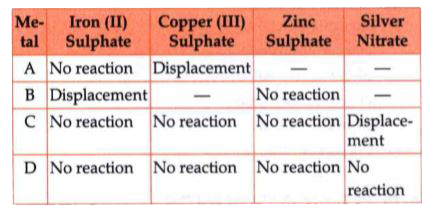
Which is the most reactive metal?
View SolutionMetal X is found in nature as its sulphide XS. It is used in the galvanisation of iron articles. Identify the metal X. How will you convert this sulphide ore into the metal? Explain with equations.
View SolutionYou are provided with 18 g each of O2,N2,CH4andH2O. Which of the following is the correct decreasing order of number of atoms present in these samples ?
View Solutionनिम्न समीकरण को पूरा करो-
C2H5OHConc.H2SO4−−−−−−−→170∘CmHot(A)O3−−→CCl4(B)Zn+H2O−−−−−→(C)
View Solutionअभिक्रिया CH3CH2OHH2SO4−−−−−−→160−170∘CCH2=CH2+H2O की क्रिया-विधि समझाइए |
View SolutionIn the reaction HCOOHH2SO4−−−→CO+H2O,H2SO4 acts as
View SolutionThe reaction CH_3CH_2OH underset"453 K"overset(95% H_2SO_4)to CH_2=CH_2+H_2O is an example of
View SolutionIn the reaction X underset(443K)overset(H_2SO_4)toY overset(HBr)toZ underset(H_2O)overset(KOH)to CH_3underset(OH)underset(|)(C)HCH_3X is
View SolutionCH_3-CH=CH_2 underset(+H_2O)overset("Conc."H_2SO_4)to P overset(PCl_3)toQ underset(KOH)overset("alcoholic")toR . The compound R is
View SolutionIn the sequence of reaction , identify (Z). CH_3CH_2CH_2OH overset(PCl_3)to X overset(alc. KOH)to Y underset(H_2O,"boil")overset("Conc."H_2SO_4)to Z
View SolutionThe reaction : CH3CH2OH95%H2SO4−−−−−−→453KCH2=CH2+H2O is an example of
View SolutionIdentify D in the following reaction series : CH3−CH=CH2Conc.H2SO4−−−−−−−→AH2O−−→ΔBPCl5−−→Calc.KOH−−−−−→D
View SolutionAK2Cr2O7−−−−−→dil.H2SO4BCH3MgI−−−−→H2OCH3−CH3∣C∣OH−CH3
The reactant A is ________.
View SolutionIn presence of few drops of conc. H_2SO_4 , the product formed in the following reaction is : H_3C-underset(CH_3)underset(|)C=CH_2+H_2O overset(H^(+))to
View Solution