Compound having the lowest dipole moment is
A
cis-2-butyne
B
2-butyne
C
1-butyne
D
H2C=CH−C≡CH
Text Solution
The correct Answer is:B
CH3−C≡C−CH3
Due to symmetric structure, internal dipole moments cancel out each other.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following compound has the lowest dipole moment
A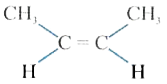 BCH3C≡CCH3CCH3CH2c≡CHDCH2=CH−C≡CH
BCH3C≡CCH3CCH3CH2c≡CHDCH2=CH−C≡CHThe compound having maximum dipole moment is
ANH3BNF3CNCl3DNI3The compound that has the highest dipole moment is
Acis-1, 2-dichloroetheneBtrans-1, 2-dichloroetheneCcis-1-bromo-2-chloroetheneDtrans-1-bromo-2-chloroethene
Which of the following hydrocarbons has the lowest dipole moment ?
View SolutionWhich of the following hydrocarbons has the lowest dipole moment ?
View SolutionWhich of the following hydrocarbons has the lowest dipole moment
View SolutionWhich of the following hydrocarbons has the lowest dipole moment?
View SolutionWhich of the following hydrocarbons has the lowest dipole moment?
View Solution

