What is the incorrect statement regarding two given complexes [Cr(H2O6)Cl3](A)and[Cr(NH3)6]Cl3(B) which are violet and yellow coloured respectively ?
Both are paramagnetic with three unpaired electrons
Δ0 value of (A) is less than that of
Δ0 values of (A) and (B) are calculated from the energies of violet and yellow light, respectively.
Both absorb energies corresponding to their complementary colors.
The correct Answer is:C
(Δ0)A<(Δ0)B both complexes have CN = 6, B has strong field ligand and A has weak field ligand
so (Δ0)A<(Δ0)B
In both complexes, Cr has 3 unpaired electrons and hence, will be paramagnetic [Cr(H2O)]Cl3
[Cr+3=1S22S2sps3S23p63d3] in strong field ligand or weak field ligand is t1112g ego∘
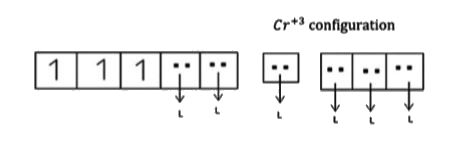
Δ0
order will be compared by spectrochemical series not by energies of violet and yellow light. Hence incorrect
Topper's Solved these Questions
Similar Questions
Two complexes [Cr(H2O6)6]Cl3 and [Cr(NH3)6]Cl3 (B) are violet and yellow coloured, respectively. The incorrect statement regarding them is :
View SolutionTwo complexes [Cr(H2O6)6]Cl3 and [Cr(NH3)6]Cl3 (B) are violet and yellow coloured, respectively. The incorrect statement regarding them is :
View Solution
Knowledge Check
Two complexes [Cr(H2O6)6]Cl3 and [Cr(NH3)6]Cl3 (B) are violet and yellow coloured, respectively. The incorrect statement regarding them is :
AΔ0 value of A is less than that of BBΔ0 value of A and B are calculated from the enrgies of violet and yellow light, respectivelyCBoth absorb energies corresponding to their complementary coloursDBoth are paramagnetic with three unpaired electronsSelect the correct statement for the complexes
[Cr(H2O)6]Cl3and[Cr(H2O)5Cl]Cl2.H2OAThere can be differentiated by amount.BThere can be diffrentiated by electrical conducting measurement method.CThere can be diffrentiated by heating with conc. H2SO4.DAll of theseWhich of the following statement is/are incorrect for the complex [Cr(H2O)6]Cl3?
AIt has a magnetic moment of 3.83 BM.BThe distribution of 3d electrons in 3dxy1,3dyz1,3dzx1CThe ligand has satisfied both primary and secondary valencies of chromium.DIt shows ionization as well as hydrate isomerism.
Two complexes [Cr(H2O6)6]Cl3 and [Cr(NH3)6]Cl3 (B) are violet and yellow coloured, respectively. The incorrect statement regarding them is :
View SolutionSelect the correct statement for the complexes
[Cr(H2O)6]Cl3and[Cr(H2O)5Cl]Cl2.H2O
View SolutionWhich of the following statement is/are incorrect for the complex [Cr(H2O)6]Cl3?
View SolutionWhich of the following statements is/are incorrect for the complex [Cr(H2O)6]Cl3?
View SolutionThe pair of complex compounds [Cr(H2O)6]Cl3 and [Cr(H2O)5Cl]Cl2H2O are an example of
View Solution

