Which of the following option is /are the correct representation of the formula for the compoud CaCl2 ?
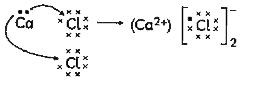
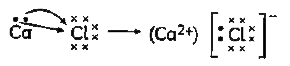
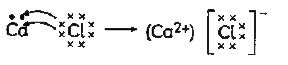
Cl-Ca-Cl
The correct Answer is:A
To determine the correct representation of the formula for the compound CaCl2, we need to analyze the components of the compound and their respective symbols.
Step 1: Identify the Elements
The compound CaCl2 consists of two elements: Calcium (Ca) and Chlorine (Cl).
Step 2: Understand the Chemical Formula
The formula CaCl2 indicates that there is one calcium atom and two chlorine atoms in each formula unit of calcium chloride.
Step 3: Valency of the Elements
- Calcium (Ca) has a valency of +2 because it is an alkaline earth metal and tends to lose two electrons.
- Chlorine (Cl) has a valency of -1 because it is a halogen and tends to gain one electron.
Step 4: Combining the Elements
To form a neutral compound, the total positive charge must balance the total negative charge. Since calcium has a +2 charge and each chlorine has a -1 charge, two chlorine atoms are needed to balance the +2 charge from one calcium atom.
Step 5: Final Representation
Thus, the correct representation of the formula for the compound is CaCl2.
Conclusion
The correct representation of the formula for the compound CaCl2 is indeed CaCl2.
---
The compound CaCl2 consists of two elements: Calcium (Ca) and Chlorine (Cl).
The formula CaCl2 indicates that there is one calcium atom and two chlorine atoms in each formula unit of calcium chloride.
- Calcium (Ca) has a valency of +2 because it is an alkaline earth metal and tends to lose two electrons.
- Chlorine (Cl) has a valency of -1 because it is a halogen and tends to gain one electron.
To form a neutral compound, the total positive charge must balance the total negative charge. Since calcium has a +2 charge and each chlorine has a -1 charge, two chlorine atoms are needed to balance the +2 charge from one calcium atom.
Thus, the correct representation of the formula for the compound is CaCl2.
The correct representation of the formula for the compound CaCl2 is indeed CaCl2.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following option is correct ?
AStenothermal organismEurythermal organismFrogLizardBStenothermal organismEurythermal organismFrogManCStenothermal organismEurythermal organismManLizardDStenothermal organismEurythermal organismPolar bearCoconut tree
Which of the following option is correct?AStenothermal organismEurythermal organismFrogLizardBStenothermal organismEurythermal organismFrogManCStenothermal organismEurythermal organismManLizardDStenothermal organismEurythermal organismPolar bearCoconut treeWhich of the following options are correct?
AMicelle formation by soap in aqueous solution is possible at all temperaturesBMicelle formation by soap in aqueous solution occurs above a particular concentrationCOn dilution of soap solution micelles may revert to individual ionsDSoap solution behaves as a normal strong electrolyte at all concentrations

 Which of the following options is correct ?
Which of the following options is correct ?