Identify the cations and anions present in aluminium oxide :
A
CationAnionAl+O−
B
CationAnionAl2+O2−
C
CationAnionAl3+O2−
D
CationAnionAl3+O−
Video Solution
Text Solution
The correct Answer is:C
Aluminium oxide or Al2O3 is formed by the transfer of electrons from aluminium atoms to oxygen atoms. Aluminium (Atomic number = 13) has 3 valence electrons whereas oxygen (atomic number=8) has 2 valence electrons.
So, each aluminium atom donates its 3 valence electrons to the oxygen atoms and forms cations [Al3+], whereas each oxygen atom gains two electrons and forms anions [O2−]
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Cation and anions are formed in
AheterolysisBhomolysisCSN2 reactionDE2 reaction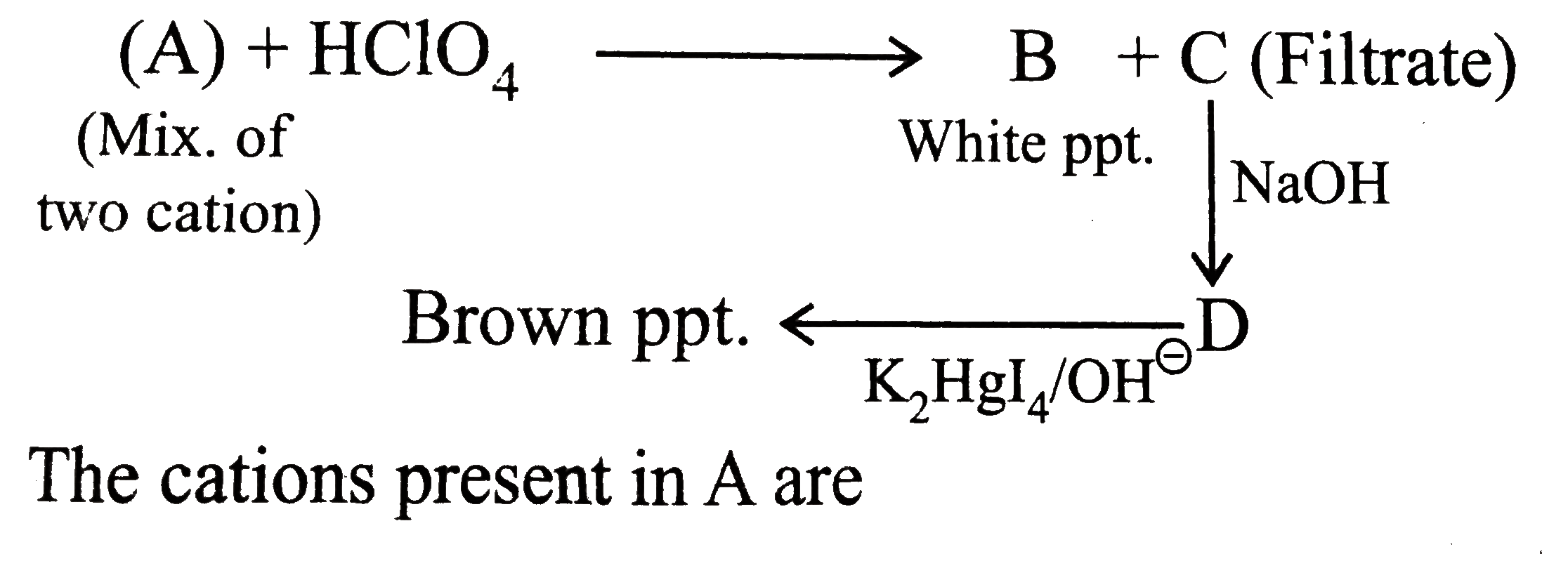
The cations present in A areAK⊕ and Na⊕BK⊕ and NH⊕4CNH⊕4 and Fe2+D(Mg)2+andNa⊕
Name the cation and anion present in MgCl2 compound.
View SolutionIdentify the cations and anions in the following compounds:
(a)CH3COONa (b)NH,3 (c )NH4CI (iv)SrCl2.
View SolutionWrite the cations and anions present (if any) in the following compounds?
a) CH3COONa
b) NaCl
c) H2
d) NH4NO3
View Solution(a). What is an ion? Explain with examples
(b). What iis the nature of charge on (i). A cation and (ii) and anion?
(c) Name the cation and anion present in MgCl2 also write their symbols.
View SolutionIdentify the cation and anion in the molecular formula of potassium sulphate?
View SolutionSelect the row containing correct cation and anion in calcium oxide:
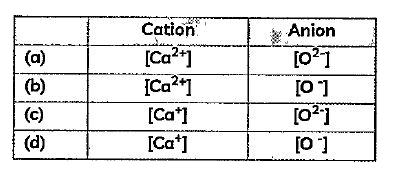
View Solution