Among the following, which is the strongest acid?
A
CH(CN)3
B
CHI3
C
CHBr3
D
CHCI3
Text Solution
The correct Answer is:A
Acidic character a stability of conjugate base (anion)
Stability of anion α M and -I
−M>−I ( dominance )
Electron withdrawing groups increase acidic strength. Among the given compounds, the electron withdrawing strength of substituents
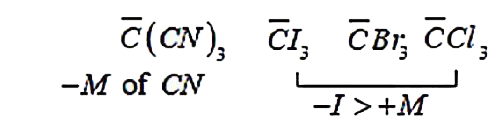
Hence, CH (CN)3 , will be most acidic
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which is the strongest Lewis acid?
ABF3BBCl3CBBr3DBI3Among the following which acid is strongest?
AAcetic acidBMonochloroacetic acidCDichloroacetic acidDTrichloroacetic acidThe strongest acid is
ACl3CCOOHBNCCH2COOHCO2NCH2COOHDF3CCOOH
Which among the following is the strongest acid ?
View SolutionAmong the following compounds, the strongest acid is :
View SolutionAmong the following compounds, the strongest acid is :
View SolutionAmong the following compounds the strongest acid is :
View SolutionAmong the following compounds, the strongest acid is "
View Solution

