A compound contains 20% sulphur. The molecular weight of the compound could be
80
240
400
640
The correct Answer is:D
To determine the molecular weight of a compound that contains 20% sulfur, we can follow these steps:
1. Understand the Percentage Composition:
The compound contains 20% sulfur by mass. This means that in 100 grams of the compound, there are 20 grams of sulfur.
2. Identify the Atomic Mass of Sulfur:
The atomic mass of sulfur (S) is approximately 32 g/mol.
3. Set Up the Equation for Percentage Composition:
The percentage of sulfur in the compound can be expressed as:
Percentage of Sulfur=(Mass of Sulfur in the compoundMolecular Weight of the Compound)×100
Plugging in the known values, we have:
20=(32M)×100
4. Rearrange the Equation:
To find the molecular weight (M), rearrange the equation:
20M=32×100
20M=3200
M=320020
M=160 g/mol
5. Determine the Molecular Weight:
The molecular weight of the compound must be an integral multiple of the empirical formula mass. Therefore, the molecular weight could be 160, 320, 480, 640, etc.
6. Check the Options:
If the options provided include 640, we can conclude that it is a valid molecular weight since it is a multiple of 160.
Final Answer:
The molecular weight of the compound could be 640 g/mol.
---
The compound contains 20% sulfur by mass. This means that in 100 grams of the compound, there are 20 grams of sulfur.
The atomic mass of sulfur (S) is approximately 32 g/mol.
The percentage of sulfur in the compound can be expressed as:
Percentage of Sulfur=(Mass of Sulfur in the compoundMolecular Weight of the Compound)×100
Plugging in the known values, we have:
20=(32M)×100
To find the molecular weight (M), rearrange the equation:
20M=32×100
20M=3200
M=320020
M=160 g/mol
The molecular weight of the compound must be an integral multiple of the empirical formula mass. Therefore, the molecular weight could be 160, 320, 480, 640, etc.
If the options provided include 640, we can conclude that it is a valid molecular weight since it is a multiple of 160.
The molecular weight of the compound could be 640 g/mol.
Similar Questions
Knowledge Check
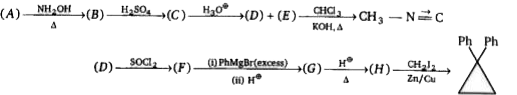
Molecular weight of compound (A) is :A58B120C60D182
An enzyme contains 2% of sulphur. The molecular weight of the Enzyme is 6400. How many sulphur atoms are present in that enzyme molecule?
View SolutionA compound was found of contain 14.34% oxygen. The minimum molecular weight of the compound is
View SolutionInsulin contains 3.2 % sulphur. The minimum molecular weight of insulin is
View SolutionFind the empirical form~a of a compound of carbon and hydrogen which contains 80% carbon. If the molecular weight of the above compound is 30, what is its molecular formula ?
View SolutionA organic compound contains 4% sulphur. Its minimum molecular weight is
View SolutionA compound contains 3.2% of oxygen. The minimum mol. Wt. of the compound is
View SolutionA compound contain 3.4% by weight of sulphur, the minimum molecular wt of compound is:-
View Solution

