The oxidation number of carbon in Freon, and hydroge cyanide is +x and +y. What is the value x+y.
The correct Answer is:6
To find the values of x and y, which represent the oxidation numbers of carbon in Freon and hydrogen cyanide respectively, we will analyze each compound step by step.
Step 1: Determine the oxidation number of carbon in Freon
1. Identify the formula of Freon: The common formula for Freon is CCl2F2.
2. Assign oxidation numbers to other elements:
- Chlorine (Cl) typically has an oxidation number of −1.
- Fluorine (F) has an oxidation number of −1.
3. Set up the equation:
- Let the oxidation number of carbon be x.
- The overall charge of the molecule is neutral, so we can write the equation:
x+2(−1)+2(−1)=0
- Simplifying this gives:
x−2−2=0⟹x−4=0⟹x=+4
Step 2: Determine the oxidation number of carbon in hydrogen cyanide
1. Identify the formula of hydrogen cyanide: The formula is HCN.
2. Assign oxidation numbers to other elements:
- Hydrogen (H) has an oxidation number of +1.
- Nitrogen (N) typically has an oxidation number of −3.
3. Set up the equation:
- Let the oxidation number of carbon be y.
- The overall charge of the molecule is neutral, so we can write the equation:
y+1+(−3)=0
- Simplifying this gives:
y+1−3=0⟹y−2=0⟹y=+2
Step 3: Calculate x+y
1. Add the values of x and y:
x+y=4+2=6
Final Answer
The value of x+y is 6.
---
1. Identify the formula of Freon: The common formula for Freon is CCl2F2.
2. Assign oxidation numbers to other elements:
- Chlorine (Cl) typically has an oxidation number of −1.
- Fluorine (F) has an oxidation number of −1.
3. Set up the equation:
- Let the oxidation number of carbon be x.
- The overall charge of the molecule is neutral, so we can write the equation:
x+2(−1)+2(−1)=0
- Simplifying this gives:
x−2−2=0⟹x−4=0⟹x=+4
1. Identify the formula of hydrogen cyanide: The formula is HCN.
2. Assign oxidation numbers to other elements:
- Hydrogen (H) has an oxidation number of +1.
- Nitrogen (N) typically has an oxidation number of −3.
3. Set up the equation:
- Let the oxidation number of carbon be y.
- The overall charge of the molecule is neutral, so we can write the equation:
y+1+(−3)=0
- Simplifying this gives:
y+1−3=0⟹y−2=0⟹y=+2
1. Add the values of x and y:
x+y=4+2=6
The value of x+y is 6.
Similar Questions
Partial reduction of Cyanides with SnCl2 / HCl gives X which on hydrolysis form Y . What is X and Y ?
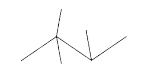
View SolutionLet number of primary carbon atoms be x, secondary carbon atoms be y in the following compound x+y is :
View Solutionএকটি আয়তঘনবস্তুর তলসংখ্যা x ও শীর্ষবিন্দুর সংখ্যা y হলে xy-এর মান কত ?
View SolutionWhat is the value of (x-y + z) if the number of angular points of a quadrilateral is x, the number of vertices is y and the number of planes is z?
View SolutionIf x=5,y=3, then what is the value of (x+y)x/y ?
View SolutionThe number of π bonds in naphthalene is y, number of σ bonds in z and number of carbon atoms is x. calculate the value of y+zx ?
View Solutionএকটি আয়তঘনের তল সংখ্যা x , ধার সংখ্যা y এবং শীর্ষবিন্দুর সংখ্যা z হলে, x+y+z এর মান কত?
View SolutionNumber of chiral carbons in (M) =X (N) [(N) in the number of geometric isomers =y] y/2 The value of :
View Solution
