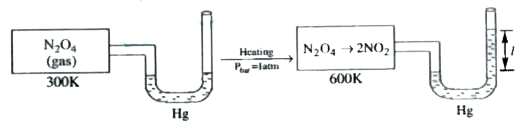
If the value of l in the above diagram is 114 cm. What is the percentage dissociation of N2O4 due to heating.
40%
50%
25%
12.5%
Step by step video solution for If the value of l in the above diagram is 114 cm. What is the percentage dissociation of N_2O_4 due to heating. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023समान प्रश्न

If the value of 7' in the above diagram is 114 cm. What is the percentage dissociation of N2O4 due to heating.
View SolutionK_p for the reaction N_2O_4(g) leftrightarrow 2NO_2(g) at 750K is 640torr. Calculate the percentage of dissociation of N_2O_4 at equilibrium pressure of 160torr.
View SolutionIf in the reaction N2O4⇌2NO2,α is degree of dissociation of N2O4 Then the number of molecules at equiliberium will be :
View SolutionThe vapour density of undecomposed N2O4 is 46. When heated vapour density decreases to 24.5 due to its dissociation to NO2 WHAT is the percent dissociation of N2O4 at the final stage ?
View Solutionकिसी ताप पर N2O4 का वाष्प घनत्व 30 है। इस ताप पर N2O4 का प्रतिशत वियोजन क्या होगा?
View SolutionN2O4 is 25% dissociated at 37∘C and 1atm. Calculate (i) Kp and (ii) the percentage dissociation at 0.1 atm and 37∘C.
View SolutionThe vapour density of undecomposed N_2O, is 46. When heated, the vapour density decreases to 24.5 due to its dissociation to NO_2. The percentage dissociation of N_2O_4 at the final temperature is
View SolutionAt constant temperature, vapour density of N_2O_4 is found to be 40. Percentage degree of dissociation of N_2O_4 at this constant temperature will be N_2O_4(g)hArr2NO_2(g)
View Solution

