What is the correct decreasing order of bond length?
O2−2<O2>O2
O2>O−2>O2−2
O−2>O2−2>O2
O−2>O2>O2−2
The correct Answer is:A
O2−2>O2>O2
टॉपर्स ने हल किए ये सवाल
समान प्रश्न
Which among the following options represents the correct order of stability:
View SolutionFind the exact number of Ione pair of electrons at the central atom in XeF4,XeO4 and XeO2F2, respectively
View SolutionCorrect order of bond length is
View SolutionCorrect decreasing order O-O bond length in O2,
View SolutionArrange the following molecules in the correct order of decreasing C-C bond length:
View SolutionCorrect order of bond length will be-
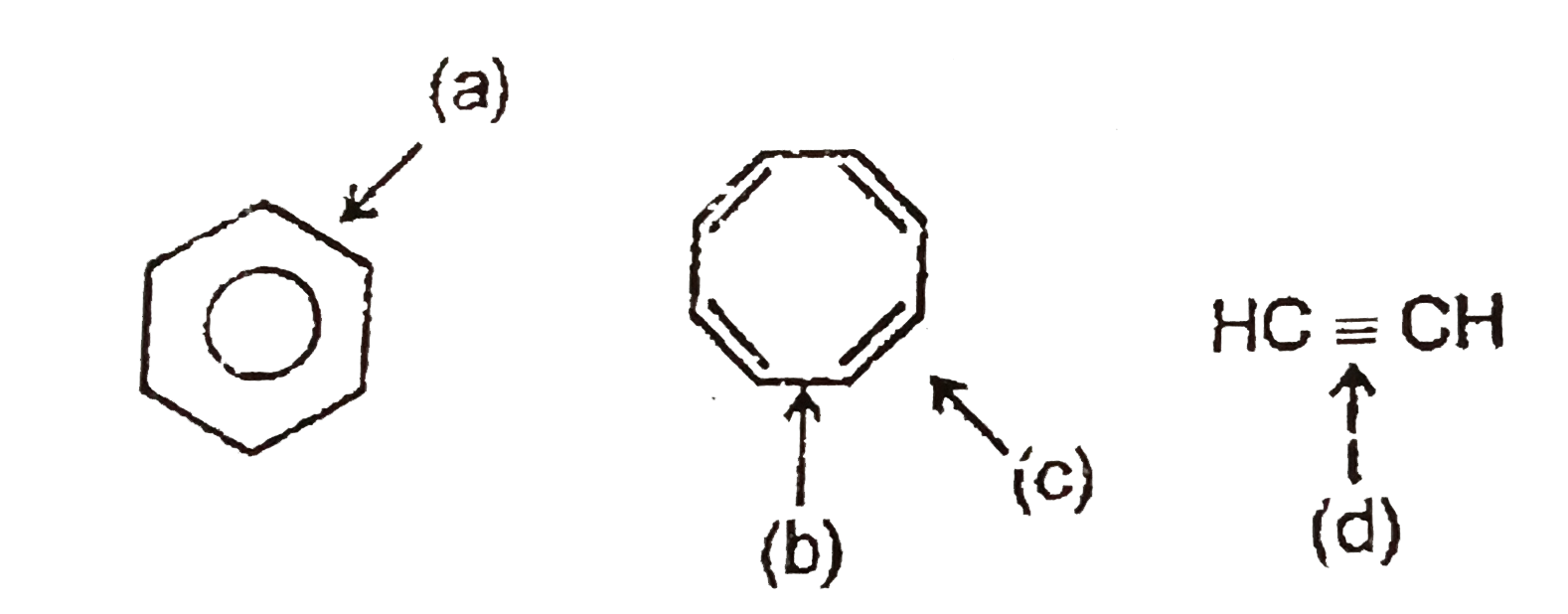
View SolutionThe decreasing order of bond length of C=C bond in the following compounds is :
View SolutionThe correct orders for bond length are :
View SolutionDoes bond length decrease as bond order increases?
View SolutionArrange the following molecules in decreasing order of bond length.
View SolutionThe correct order of bond length is
View Solutionनिम्न में से कौन बंध कोण का सही घटता हुआ क्रम है
View SolutionThe correct order of bond lengths is
View SolutionThe correct order of bond length is
View SolutionThe correct order of decreasing length of the bond as indicated by the arrow in the following structures is
View SolutionThe decreasing order of bond length of C=C bond in the following compounds is :
View Solution

