From kinetic theory of gases, show that Moon cannot have an atmosphere (Assume k=1.38×10−23JK−1 Temperature T=0∘C=273K)
vescape=vrms=1.86kms−1.
Topper's Solved these Questions
- KINETIC THEORY OF GASESBOOK - NCERT TAMILCHAPTER - KINETIC THEORY OF GASESEXERCISE - EVALUATION (MULTIPLE CHOICE QUESTIONS)
15 Videos
- KINETIC THEORYBOOK - NCERT TAMILCHAPTER - KINETIC THEORYEXERCISE - ADDITIONAL EXERCISE
4 Videos
- LAW OF MOTIONBOOK - NCERT TAMILCHAPTER - LAW OF MOTIONEXERCISE - EXERCISE (ADDITIONAL EXERCISE)
17 Videos
Similar Questions
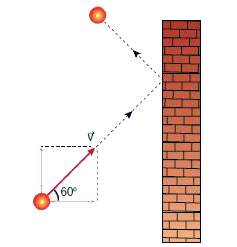
A particle of mass m is moving with speed u in a direction which makes 60∘ with respect to x axis. It undergoes elastic collision with the wall. What is the change in momentum in x and y direction ?
View SolutionA metre long narrow bore held horizontally (and close at one end) contains a 76 cm long mercury thread, which traps a 15 cm column of air. What happens if the tube is held vertically with the open end at the bottom?
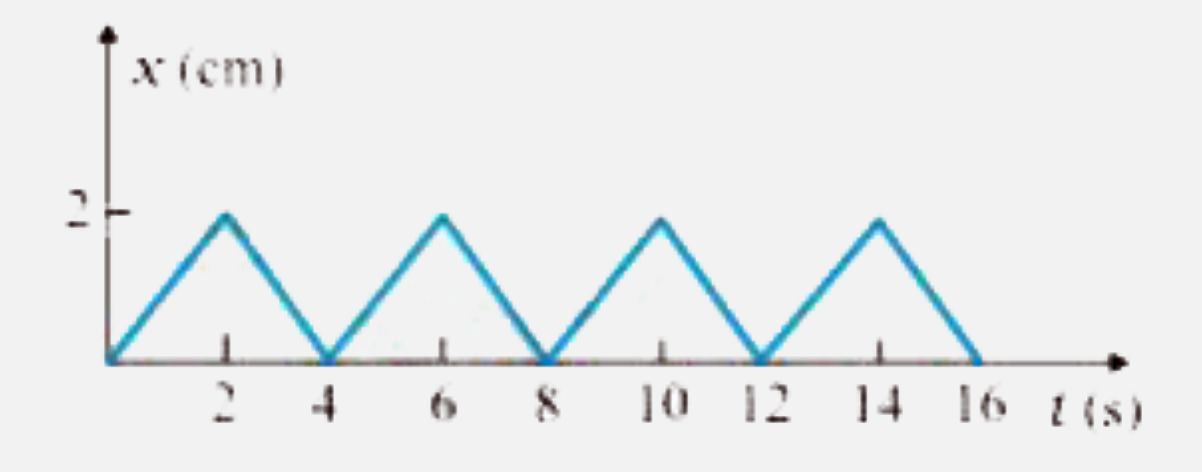
View SolutionFigure shows the position-time graph of a body of mass 0.04 kg. Suggest a suitable physical context for this motion. What is the time between two consecutive impulses received by the body ? What is the magnitude of each impulse ?
View SolutionAt what temperature does the average translational kinetic energy of molecule in a gas become equal to kinetic energy of an electron accelerated from rest through a potential difference of 1 volt? (K=1.38×10−23J/k)
View SolutionThe kinetic theory of gases assumes all of the following except:
View SolutionIn kinetic theory of gases, it is assumed that molecular collisions are
View Solutionचन्द्रमा पर वायुमण्डल नहीं हो सकता , क्यों ?
View Solution(A) किस ताप पर किसी गैस के अणु की औसत स्थनान्तरीय गतिज ऊर्जा 1V विभवान्तर से त्वरित इलेक्ट्रॉन की गतिज ऊर्जा के बराबर होगी?(k=1.38×10−23 जूल/K)
(B) उस ताप की गणना कीजिए जिस पर किसी गेस के एक अणु की माध्य गतिज ऊर्जा 4.2×10−19 जूल होगी। (k=1.38×10−23जूल/K)
View SolutionCalculate the average kinetic energy of hydrogen molecule at 0^(@)C . Given k_(B)=1.38xx10^(-23)JK^(-1) .
View SolutionCalculate the average kinetic energy of neon molecule at 27^(@)C . Given k_(B)=1.38xx10^(-23)JK^(-1)
View SolutionValue of ____________is 1.38×10−23JK−1
View Solution1.38×10−23JK−1: Boltzmann Constant :: ___________: universal gas constant
View Solutionसूर्य की सतह के ताप (6000 K)पर हीलियम गैस की माध्य गतिज ऊर्जा ज्ञात कीजिए ( k=1.38×10−23जूल केल्विन−1 )
View SolutionFrom kinetic theory of gases, show that Moon cannot have atmosphere (Assume k=1.38xx10^(-23)JK^(-1),T0^@C=273K) .
View SolutionFrom kinetic theory of gases, show that Moon cannot have atmosphere (Assume k=1.38×10−23JK−1,T0∘C=273K).
View SolutionFrom kinetic theory of gases, show that Moon cannot have atmosphere (Assume k=1.38×10−23JK−1,T0∘C=273K).
View Solution

