Among the following which will have the least hindered (restricted) rotation about carbon carbon sigma and pi bond?
Acetylene
Hexachloroethane
Ethane
Ethylene
The correct Answer is:C
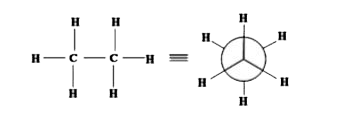
Rotation around the C-C bond in ethylene C2H4 and acetylene C2H2 is not possible because of the presence of double and triple bond. Among ethane and hexachloroethane, ethane has the smallest sized group (H) bonded to carbons hence, there will be least hindered rotation about C-C bond.
Topper's Solved these Questions
Similar Questions
Which of the following molecule is paramagnetic?
View SolutionAccording to the VSEPR theory the geometry of ClO−3 ion should be
View SolutionWhich of the following will have the least hindered rotation about carbon-carbon bonds?
View SolutionWhich of the following will have least hindered rotation about carbon-carbon bond?
View SolutionThe restricted rotation about carbon-carbon double bond in 2-butene is due to
View SolutionWhich of the following will have the least hindered rotation about carbon-carbon bonds?
View SolutionWhich of the following will have least hindered rotation about carbon-carbon bond ?
View SolutionWhich of the following will have least hindered rotation around carbon- carbon bond ?
View SolutionWhich of the following will have least hindered rotation abou carbon -carbon bond?
View SolutionExplain why rotation about carbon-carbon double bond is hindered ?
View SolutionWhich of the following will have least hindered rotation about carbon-carbon bond?
View SolutionWhich of the following will have least hindered rotation about carbon-carbon bond ?
View SolutionWhich of the following will have least hindered rotation about carbon-carbon bond ?
View SolutionThe restricted rotation about carbon-carbon double bond in 2-butene is due to
View SolutionWhich of the following has no hindered rotation about carbon-carbon bond?
View SolutionWhich of the following will have least hindered rotation about carbon -carbon bond?
View Solution

