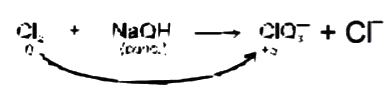When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from:
zero to +1 and zero to −5
zero to -1 and zero to +5
zero to -1 and zero to +3
zero to +1 and zero to −3
The correct Answer is:B
Topper's Solved these Questions
Similar Questions
The basic geometry and shape of I−3 are respectively:
View SolutionWhat is the correct order of increasing C-O bond lengths of CO,CO−23 and CO2?
View SolutionWhen Cl_(2) gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
View SolutionWhen CI_(2) gas reacts with hot and concentrated sodium hydroxide solution ,the oxidation number of chlorine changes from:
View SolutionWhen CI2 gas reacts wioth hot and concentrated solution of sodium hydroxide the oxidation number of chloirine changes from:
View Solutionक्लोरीन की ठण्डे व तनु एवं गर्म व सान्द्र सोडियम हाइड्रॉक्साइड विलयन से क्रिया होती है |
View Solutionजब CI2 और गर्म तथा सान्द्र सोडियम हाइड्रोऑक्साइड विलयन के साथ अभिक्रिया करती है, तो क्लोरीन की ऑक्सीकरण संख्या में परिवर्तन होता है :
View SolutionWhen Cl_(2) gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from :
View SolutionWhen Cl_(2) gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
View SolutionWhen CI2 gas reacts with hot and concentrated sodium hydroxide solution ,the oxidation number of chlorine changes from:
View SolutionWhen CI_(2) gas reacts with hot and concentrated sodium hydroxide solution the oxidation number of chlorine changes from
View SolutionHow hot and concentrated sodium hydroxide reacts with chlorine gas ?
View Solutionगर्म तथा सान्द्र सोडियम हाइड्रॉक्साइड के क्लोरीन की अभिक्रिया देती है
View Solutionगर्म तथा सान्द्र सोडियम हाइड्रॉक्साइड के क्लोरीन की अभिक्रिया देती है
View Solutionगर्म तथा सान्द सोडियम हाइड्रॉक्साइड के क्लोरीन की अभिक्रिया देती है
View SolutionWhen Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
View Solution