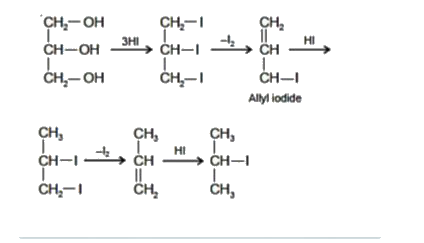
When glycerol reacts with exactly 3 moles of HI, it forms X and when it reacts with an excess of HI, it forms Y. Here Yand X, respectively are:
2-iodopropane andallyl iodide
1-iodopropane andallyl iodide
Allyl iodide and 2-iodopropane
Alyl iodide and propene
The correct Answer is:A
Topper's Solved these Questions
Similar Questions
Consider the following statements and give the answer.
Statement 1: POF3 exist but NOF3
does not exist.
Statement 2: P cannot form five bond by expanidng its octet while N can expand its octet to form five bonds
View SolutionWhat is the formal charge on SnCl−3 in ion?
View SolutionWhich product is formed when
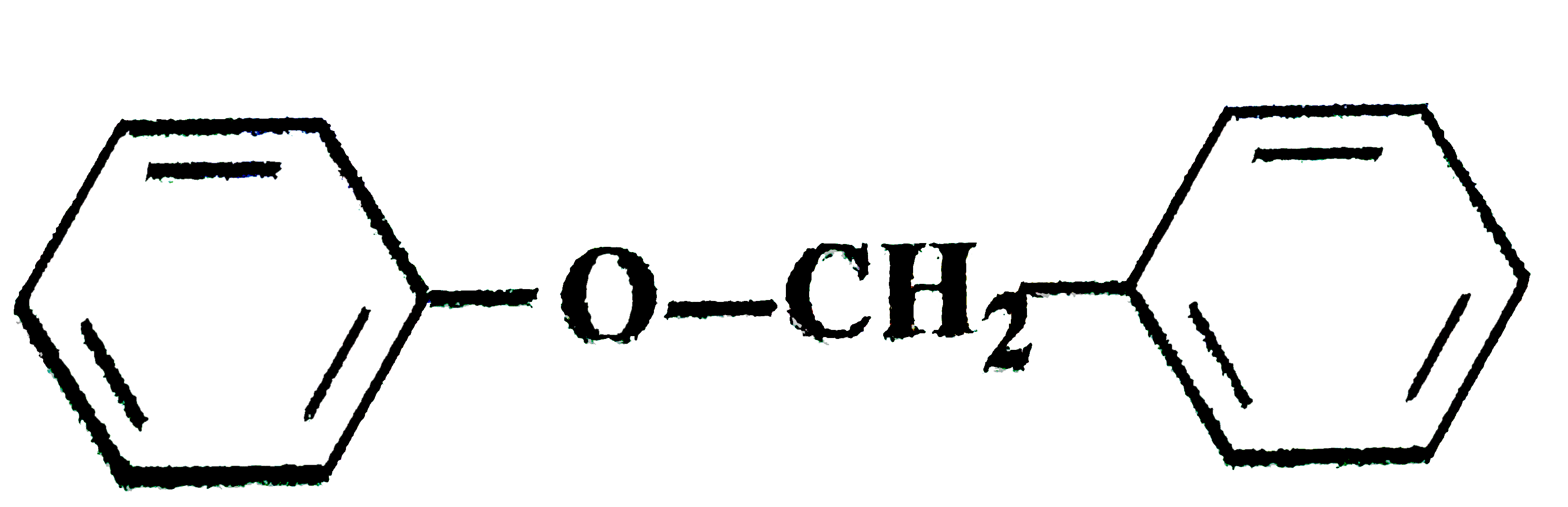 is reacted with HI ?
is reacted with HI ?
View SolutionWhich of the following is not formed when glycerol reacts with HI ?
View SolutionWhat is formed when glycerol reacts with HIO4 ?
View SolutionOne mole of an organic compound A with the formula C3H8O reacts completely with two moles of HI to form X and Y. When Y is boiled with aquous alkali it forms Z.Z answers the iodoform test. The compound A is
View SolutionTetrahydrofuran reacts with excess of HI to form-
View SolutionChloroethene reacts with Y to form NaCl and Z. One mole of Z reacts with two moles of HI to form water and iodo ethene. Which of the following is Y?
View SolutionChloroethene reacts with Y to form NaCl and Z. One mole of Z reacts with two moles of HI to form water and iodo ethene. Which of the following is Y?
View Solutionग्लिसरॉल को HI के आधिक्य के साथ अभिकृत करने पर प्राप्त होता है
View SolutionBF_(3) reacts with NaH at 450 K to form NaF and X. When X reacts with LiH in diethyl ether, Y is formed. What is Y?
View SolutionWhen CH2=CH−O−CH2−CH3 reacts with one mole of HI , one of the products formed is
View Solutionजब ग्लिसरॉल HI के साथ अभिक्रिया करता है तो क्या बनता है
View SolutionHow many moles of Hi reacts with glycerol to give 2 iodopropane?
View SolutionHow many moles of Hi reacts with glycerol to give 2 iodopropane?
View SolutionGlycerol reacts with 3 moles of HI to form 'X' and excess of HI to form Y. Here 'Y' and 'X' are respectively
View Solution