Choose the correct answer from the options given below
An electrolyte which completely dissociates into ions is :
Alcohol
Carbonic acid
Sucrose
Sodium hydroxide
The correct Answer is:D
When we dissolve Sodium hydroxide (NaOH) in water, it separates into two positively charged sodium ions and negatively charged hydroxide ions.
Topper's Solved these Questions
- ELECTROLYSISBOOK - ICSECHAPTER - ELECTROLYSISEXERCISE - Reason Based Questions
6 Videos
- ELECTROLYSISBOOK - ICSECHAPTER - ELECTROLYSISEXERCISE - Figure Based Questions
8 Videos
- ELECTROLYSISBOOK - ICSECHAPTER - ELECTROLYSISEXERCISE - Fill in the Blanks
22 Videos
- CHEMISTRY-2015BOOK - ICSECHAPTER - CHEMISTRY-2015EXERCISE - SECTION-II (40 Marks) Attempt any four questions from this Section.
49 Videos
- METALLURGYBOOK - ICSECHAPTER - METALLURGYEXERCISE - UNIT TEST PAPER 6 - Metallurgy (Select the correct answer from the list A,B,C & given in each statement.)
5 Videos
Similar Questions
Metals like potassium, calcium, sodium, etc., can be extracted only by electrolysis. Why?
View SolutionStudy the given figure and answer the question that follow :
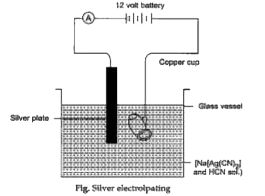
Name the cathode and anode used during electroplating of silver.
View SolutionElectrolysis is the passage of ........... through a liquid or a solution accompanied by a ...... change.
View SolutionArrange the following as per the instructions given in the brackets:
Cs, Na, Li, K, Rb (increasing order of metallic character).
View SolutionThe oxide of the metal which reacts with both acids and alkalis-to give salt & water
(a) MgO
(b) CuO
(c) Al2O3
(d) K2O
View SolutionStudy the following figure and choose the correct options. Assuming complete dissociation of electrolyte:
View SolutionMatch the following columns and choose the correct answer from the option given below.

View SolutionMatch the following columns and choose the correct answer from the option given below.
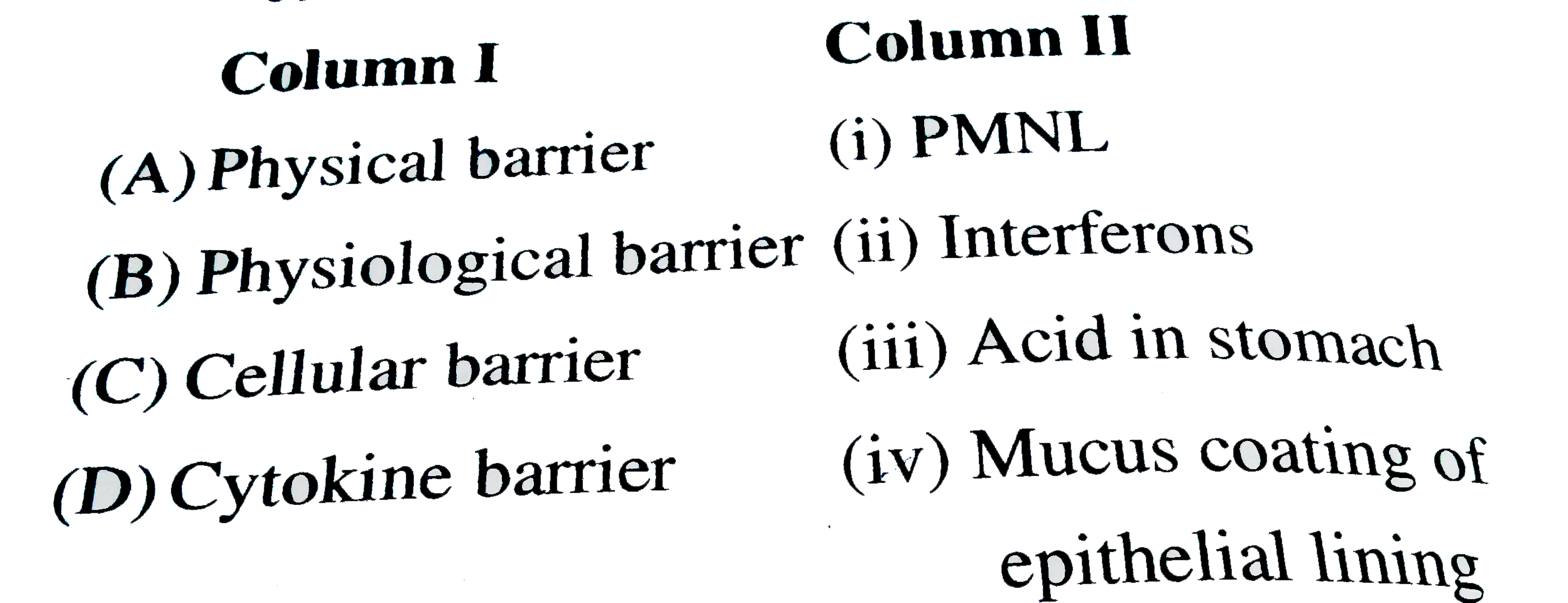
View SolutionMatch the following columns and choose the correct answer from the options given below -
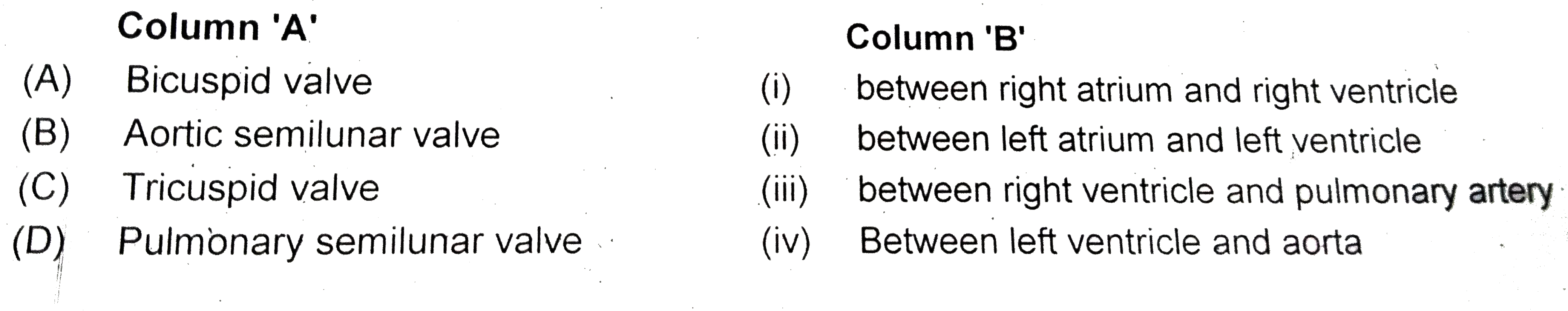
View SolutionMatch the following columns and choose the correct answer from the options given below

View SolutionStudy the following figure and choose the correct options from the given below (assuming both dissociate completely).
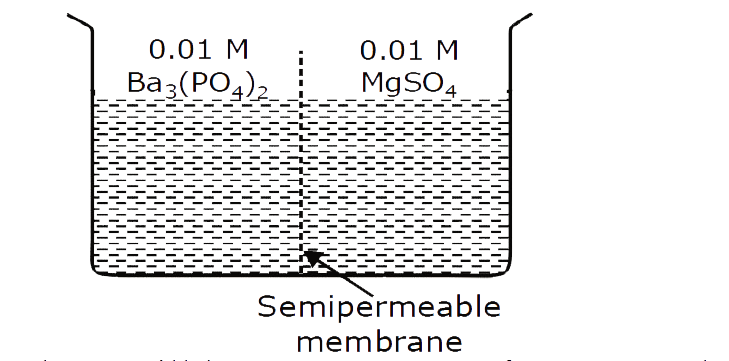
View SolutionStudy the following figure and choose the correct options. Assuming complete dissociation of electrolyte:
View SolutionMatch the columns and choose the correct answer from the options given below
View SolutionMatch the columns and choose the correct answer from the options given below
View SolutionMatch the columns and choose the correct answer from the options given below
View SolutionMatch the columns and choose the correct answer from the options given below
View Solution
