One mole of an ideal gas goes from an initial state A to final state B via two processs : It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P−V diagram representing the two process in (figure)




The correct Answer is:B
For isothermal expansion P-V curve is rectangular hyperbola (clockwise curve)
Topper's Solved these Questions
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQs
Explore 613 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:QUESTION
Explore 83 Videos
- MOTION IN TWO AND THREE DIMENSIONBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:MOTION IN TWO AND THREE DIMENSIONExercise:All Questions
Explore 47 Videos
- NEET 2020Book:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEET 2020Exercise:All Questions
Explore 45 Videos
Similar Questions
Two masses as shown are suspended from a massless pulley. Calculate the acceleration of the system when masses aere left free:
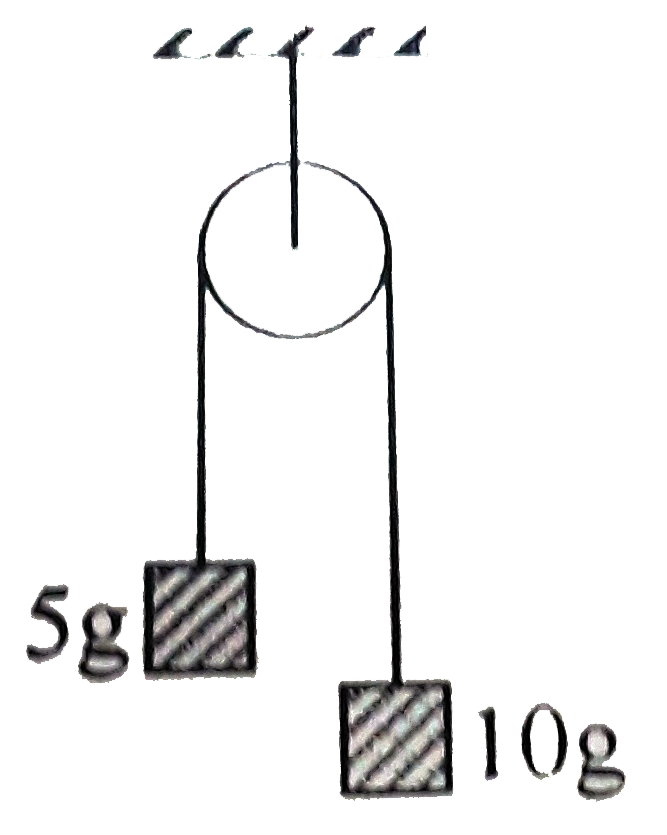
View SolutionThe mass of a .73Li nucleus is 0.042u less than the sum of the masses of all its nucleons. The binding energy per nucleon of .73Li nucleus is nearly
View SolutionThe x and y coordinates of the particle at any time are x=5t−2t2andy=10t respectively, where x and y are in meters and t in seconds. The acceleration of the particle at t=2s is:
View SolutionLight with an average flux of 20Wcm2 falls on a non-reflecting surface at normal incidence having surface area 20cm2. The energy received by the surface during time span of 1 minute is:
View SolutionOne mole of an ideal gas goes from an initial state A to final state B via two processs : It first undergoes isothermal expansion from volume V to 3 V and then its volume is reduced from 3 V to V at constant pressure. The correct P-V diagram representing the two process in (figure)
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
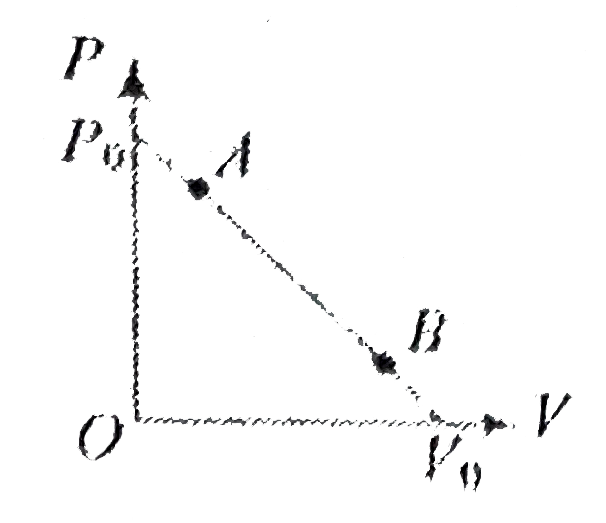
The absolute temperature T versus volume V for the given process is
View SolutionOne mole of an ideal monotomic gas undergoes a linear process from A to B in which its pressure p and its volume V change as shown in figure.
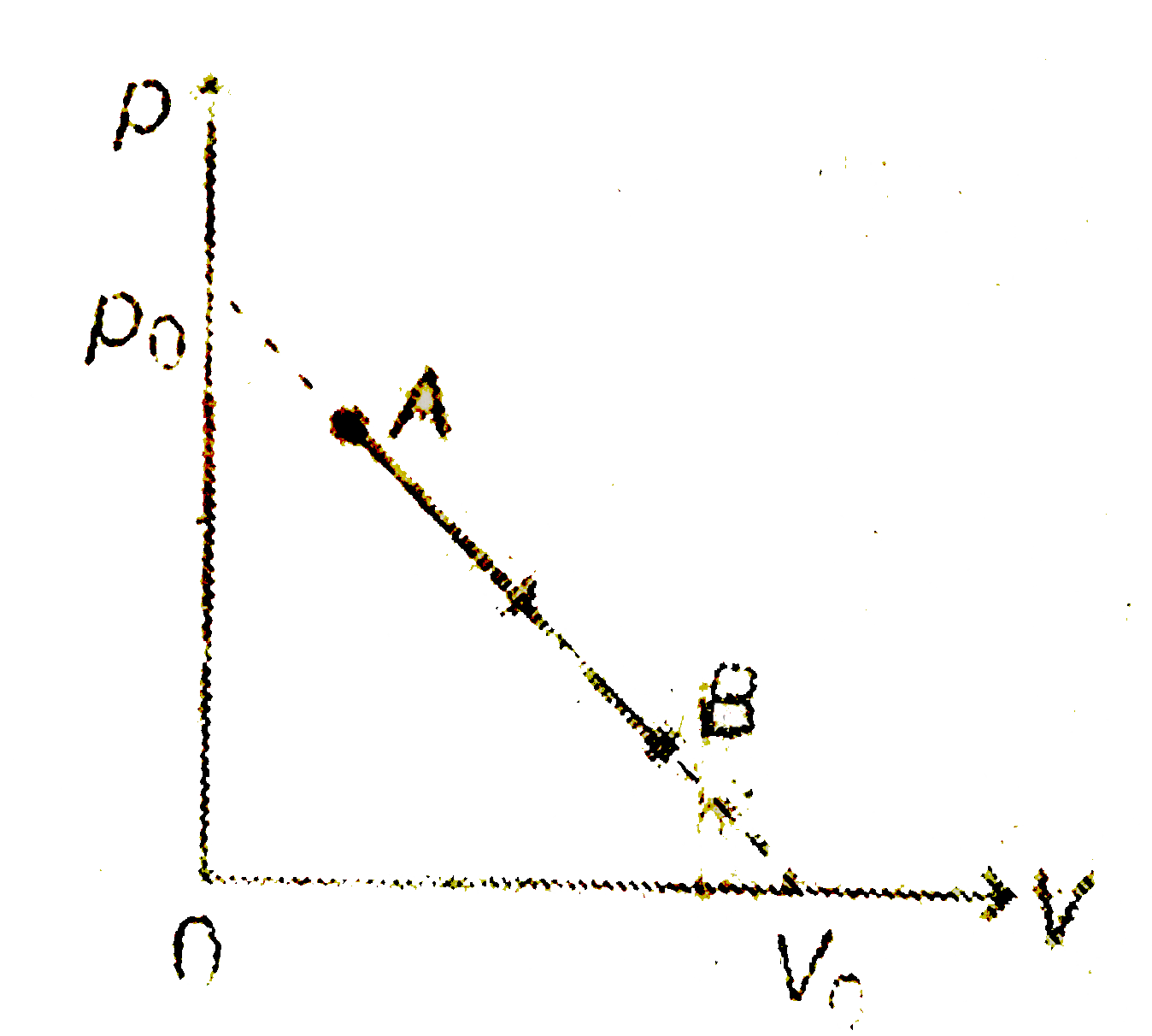
The absolute temperature T versus volume V for the given process is
View SolutionP - V diagram of an ideal gas is shown. The gas undergoes from initial state A to final state B such that initial and final volumes are same. Select the correct alternative for given process AB.
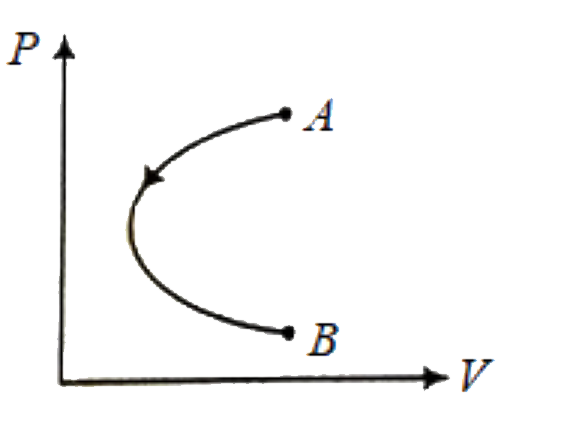
View SolutionAn ideal gas of volume V and pressure P expands isothermally to volume 16 V and then compressed adiabatically to volume V . The final pressure of gas is [γ=1.5]
View SolutionOne mole of an ideal gas goes from an initial state A to final state B via two processs : It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P−V diagram representing the two process in (figure)
View SolutionOne mole of an ideal gas goes from an initial state A to final state B via two processes: It first undergoes isothemal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The cormect P-V diagram representing the two processes is :
View Solutionएक आदर्श गैस, प्रारंभिक अवस्था A से अन्तिम अवस्था B को निम्नलिखित दो प्रक्रमों से होकर जाती है। पहले इसके आयतन का V से 3V तक समतापीय रूप से प्रसार होता है। फिर, स्थिर दाब पर इसका आयतन 3V से V तक कम किया जाता है तो, इन दो प्रक्रमों को निरूपित करने के लिये सही P-V आरेख है
View SolutionOne mole of an ideal gas goes from an initial state A to final state B via two processes : It first undergoes isothermal expansion from volume V to 3V then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is
View Solutionएक आदर्श गैस प्रारंभिक अवस्थाA से अन्तिम अवस्था B को निम्नलिखित दो प्रक्रमों से होकर जाती है। पहले इसके आयतन काV से 3V तक समतापीय रूप से प्रसार होता है। फिर नियत दाब पर इसका आयतन 3V से V तक कम किया जाता है तो, इन दो प्रक्रमों को निरूपित करने के लिये सही P-V आरेख है :-
View SolutionOne mole of an ideal gas goes from an initial state A to final state B via two processs : It first undergoes isothermal expansion from volume V to 3 V and then its volume is reduced from 3 V to V at constant pressure. The correct P-V diagram representing the two process in (figure)
View Solutionएक मोल आदर्श गैस दो प्रक्रमों के माध्यम से प्रारंभिक अवस्था A से अंतिम अवस्था B तक जाती है: यह सर्वप्रथम आयतन V से 3V तक समतापीय प्रसार सम्पन्न करती है तथा फिर इसका आयतन नियत दाब पर 3Vसे Vतक कम हो जाता हैं। दो प्रक्रमों को प्रदर्शित करने वाला सही P- V ग्राफ है
View Solution
