During an adiabatic process, the pressure of gas is found to be proportional to the cube of its absolute temperature. The ratio of (Cp,m/Cv,m) for gas is :
43
2
53
32
The correct Answer is:D
In adiabatic process
P∝Tγ/γ−1
andP∝T3
γγ−1=3
∴γ=3γ−3
−2γ=−3
γ=3/2
Topper's Solved these Questions
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:MCQs
Explore 613 Videos
- NEETBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEETExercise:QUESTION
Explore 83 Videos
- MOTION IN TWO AND THREE DIMENSIONBook:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:MOTION IN TWO AND THREE DIMENSIONExercise:All Questions
Explore 47 Videos
- NEET 2020Book:NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)Chapter:NEET 2020Exercise:All Questions
Explore 45 Videos
Similar Questions
Two masses as shown are suspended from a massless pulley. Calculate the acceleration of the system when masses aere left free:
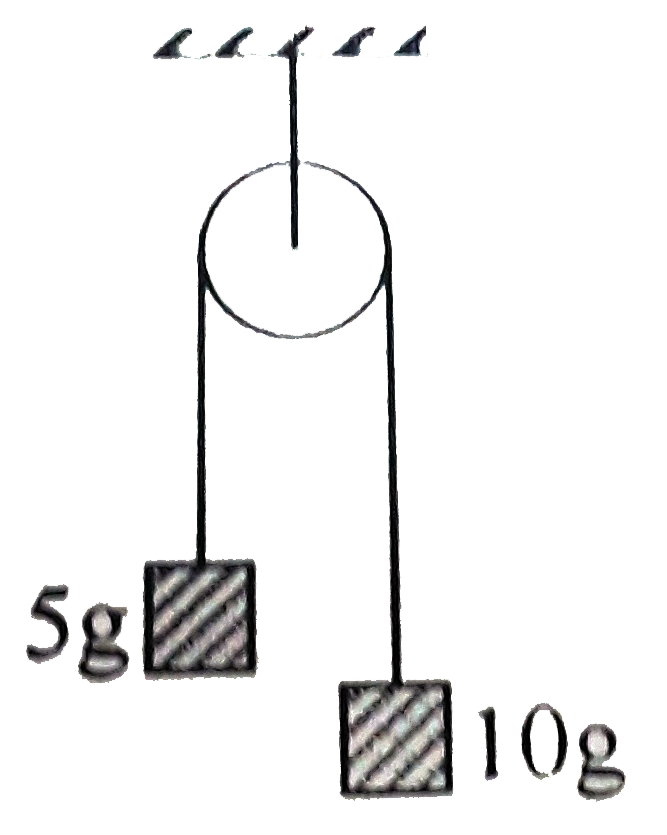
View SolutionThe mass of a .73Li nucleus is 0.042u less than the sum of the masses of all its nucleons. The binding energy per nucleon of .73Li nucleus is nearly
View SolutionThe x and y coordinates of the particle at any time are x=5t−2t2andy=10t respectively, where x and y are in meters and t in seconds. The acceleration of the particle at t=2s is:
View SolutionLight with an average flux of 20Wcm2 falls on a non-reflecting surface at normal incidence having surface area 20cm2. The energy received by the surface during time span of 1 minute is:
View SolutionDuring an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature the ratio CpCv for the gas is
View SolutionDuring an adiabatic process , the pressure of a gas is proportional to the cube of its temperature . The value of C_(I)//C_(v) for that gas is
View SolutionDuring a reversible adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio CPCV for the gas is-
View SolutionDuring an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature the ratio CpCv for the gas is
View SolutionDuring an adiabatic process, the pressure of a gas is found to be proportional to be the cubic of its absolute temperature. The ratio C_(P)//C_(V) gas is
View SolutionDuring an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature the ratio CpCv for the gas is
View SolutionDuring reversible adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature.The ratio CpCv for the gas is
View SolutionDuring an adiabatic process,the pressure of gas is found to be proportional to the cube of its absolute temperature.The ratio of C_(p)*m/C_m for gas
View SolutionDuring a reversible adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio CPCV for the gas is
View SolutionDuring an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio CP/CV for gas is
View SolutionDuring an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of CPCV for the gas is
View SolutionDuring an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio CpCv=γ for the gas is
View Solution
