A metallic crystal cystallizes into a lattice containing a sequence of layers ABABAB…. Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty spece?
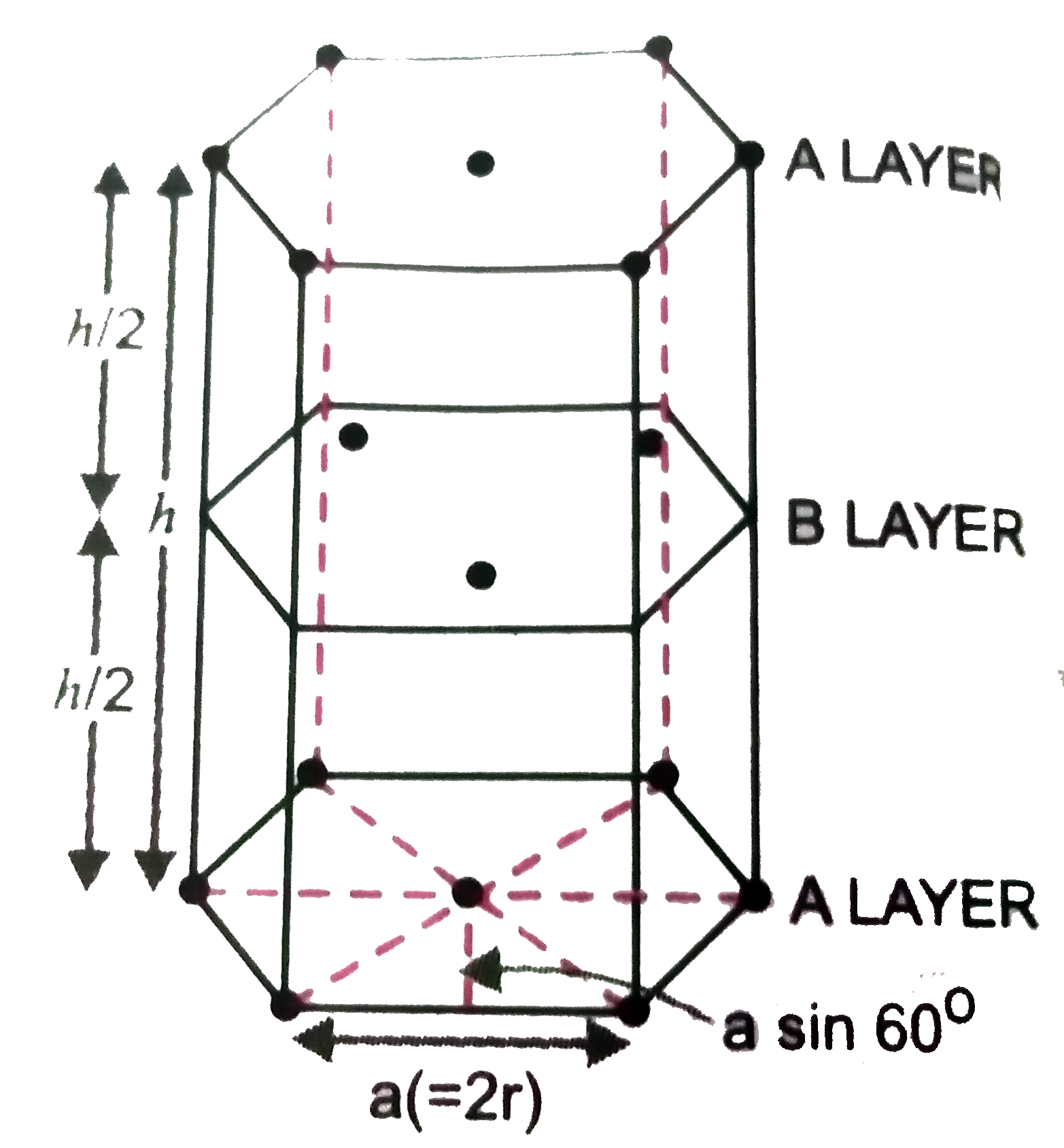
ABAB….. Type of packing means hexagonal close packing. A unit cell ( with lifted layers) is shown in the fig 1.61.
suppose the radius of each sphere =r
Volume of the unit cell = Base area × height (h)
Base area of regular hexagon = Area of six equilateral triangle s.
each with side a ( i.e, 2r) =6×√34a2
Height h =2 × Distance between closest packed layers.
2×√23a
voluvme of unit cell =(6×√34a2)(2×√23a)=3√2a3
putting a = 2r, volume of the unit cell =3√2(2r)3=24√2r3
No. of atoms in hcp per unit cell =12×16 (corners) +2×12 ( face centres) + 3( in the body) = 6
Volume of six spheres =6×43πr3=8πr3
packing fraction = 8πr324√2r3=π3√2=3.1433×1.414=0.74
% volume occupied = 74 %
% volume empty space = 26 %
Topper's Solved these Questions
- SOLID STATE BOOK - PRADEEPCHAPTER - SOLID STATE EXERCISE - Problem For Pactice
6 Videos
- S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS) BOOK - PRADEEPCHAPTER - S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS) EXERCISE - Assertion -Reason Type Question (Type 2)
19 Videos
- SOME p-BLOCK ELEMENTSBOOK - PRADEEPCHAPTER - SOME p-BLOCK ELEMENTSEXERCISE - Competition Focus (JEE( Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions (Type I)
23 Videos
Similar Questions
A compound formed by elements X and Y crysstallixzes in the cubic struture where Y atoms are at the corners of the cube and X atoms are at the alterante faces. What is the formula of the compound. ?
View SolutionAssertion (A): Alkali metals impart colour to the flame.
Reason (R ): The ionisation energies are low.
View SolutionAssertion: Boron always froms covalent bond.
Reason: The small size of B3+ favours formation of covalent bond.
View SolutionA metallic crystal cystallizes into a lattice containing a sequence of layers ABABAB…. Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty spece?
View SolutionA metallic element crystallizes into a lattice contained sequence of layers ABABAB…. Any packing of sphere leaves out voilds in the lattice.The percentage by volume of this lattice as empty space is
View SolutionA crystal made of metal crystallises into a lattice containing a sequence of layer ABABABA…. Packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is occupied ?
View SolutionA metallic element crystallizes into a lattice contained sequence of layers ABABAB…. Any packing of sphere leaves out voilds in the lattice.The percentage by volume of this lattice as empty space is
View SolutionA metallic crystal cystallizes into a lattice containing a sequence of layers ABABAB…. Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty spece?
View SolutionA metallic element crystallizes into a lattice containing a sequence of layers of ABABAB "…............" . Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty space ?
View SolutionA metallic element crystallises into a lattice containing a sequence of layers of ABABAB... Any packing of spheres leaves out voids in the lattice. Volume percentage of empty space is
View SolutionA metallic crystal cystallizes into a lattice containing a sequence of layers ABABAB…. Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty spece?
View SolutionA metallic crystal cystallizes into a lattice containing a sequence of layers ABABAB…. Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty spece?
View SolutionA metallic crystal cystallizes into a lattice containing a sequence of layers ABABAB…. Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty spece?
View SolutionA metallic element crystallises into a three dimensional lattice containing a sequence of layers of ABABAB... Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty space?
View SolutionA crystal made of metal crystallises into a lattice containing a sequence of layers ABABABA... Packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is occupied?
View Solution

