In NaCl, Cl− ions are present in the ……. Structure whereae Cl− ions ar present in the …….. Voids.
Video Solution
Text Solution
The correct Answer is:fcc, octahedral
|
Answer
Step by step video & text solution for In NaCl, Cl^(-) ions are present in the ……. Structure whereae Cl^(-) ions ar present in the …….. Voids. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STATES OF MATTER: SOLID MATTERBOOK - PRADEEPCHAPTER - STATES OF MATTER: SOLID MATTEREXERCISE - CONCEPTUAL
25 Videos
- STATES OF MATTER: SOLID MATTERBOOK - PRADEEPCHAPTER - STATES OF MATTER: SOLID MATTEREXERCISE - NCERT
49 Videos
- STATES OF MATTER: SOLID MATTERBOOK - PRADEEPCHAPTER - STATES OF MATTER: SOLID MATTEREXERCISE - TEST YOUR GRIP (MULTIPLE CHOICE)
17 Videos
- STATES OF MATTER : GASES AND LIQUIDESBOOK - PRADEEPCHAPTER - STATES OF MATTER : GASES AND LIQUIDESEXERCISE - VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)
12 Videos
- STRUCTURE OF ATOMBOOK - PRADEEPCHAPTER - STRUCTURE OF ATOMEXERCISE - Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))
12 Videos
Similar Questions
Knowledge Check
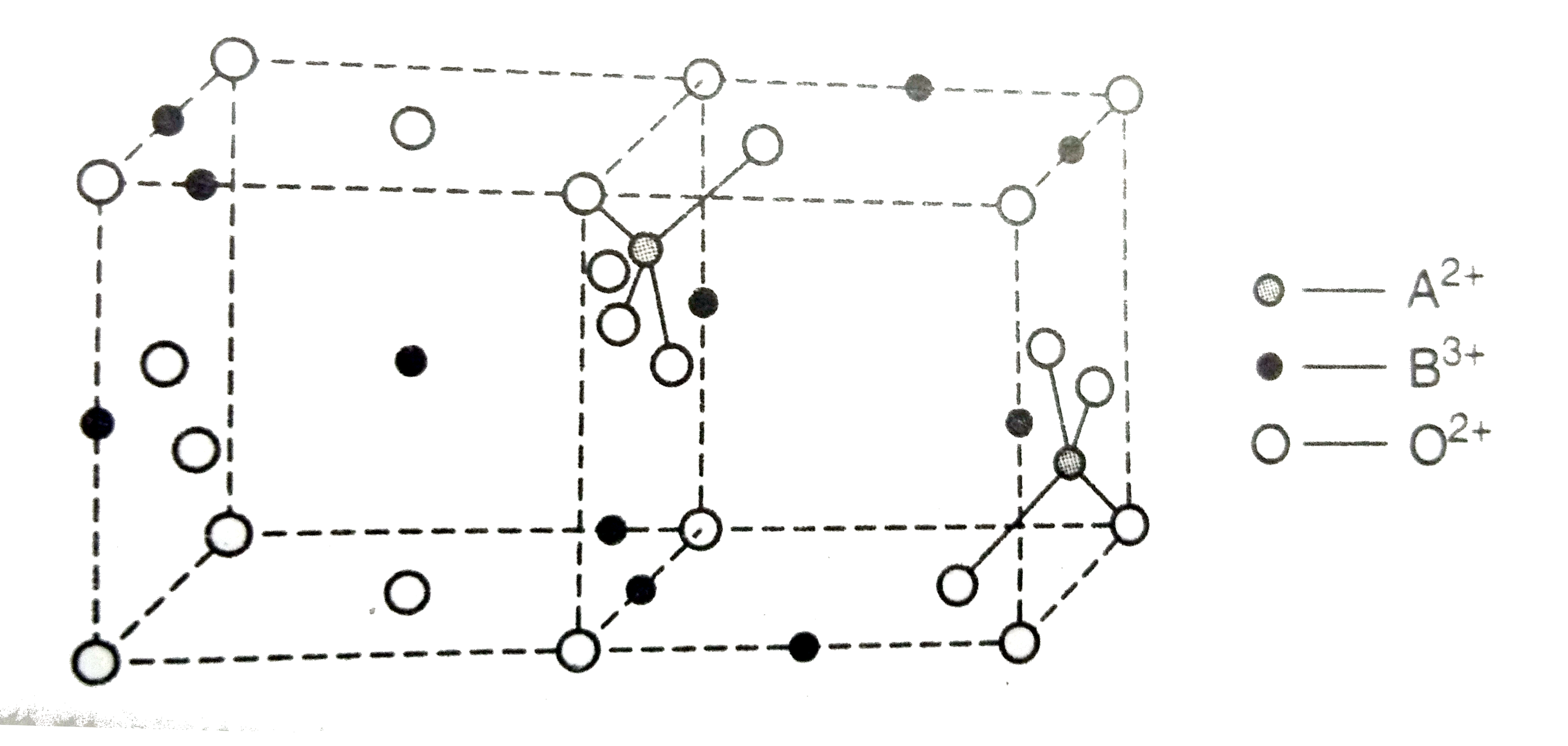
O2− ions are present in :Abcc arrangementBfcc arrangementChcp arrangementDsimple cubic arrangementThe bonds present in the structure of dichromate ion are
Aforu equivalent Cr-O bonds onlyBsix equivalent Cr-O bonds and one O -O bondCsix equivalent Cr-O bonds and one Cr-Cr bondDsix equivalent Cr-O bonds and one Cr- O -Cr bond.The bonds present in the structure of dichromate ion are
Afour equivalent Cr-O bondsBsix equivalent Cr-O bonds and one O-O bondCsix equivalent Cr-O bonds and one Cr-Cr bondDsix equivalent Cr-O bonds and one Cr-O-Cr bond
In ZnS ,S2− ions form ……. Structure while Zn2+ ions are present in ……. Voids.
View SolutionIn fluorite (CaF2),Ca2+ ions form the ……. Struture whereas F− ions are present in the ……… voids.
View SolutionName the ions present in this compound
View SolutionWhat are the ions present in these compounds?
View SolutionPeroxide ion is present in :
View Solution

