In each of the compounds : NaCl, ZnS and CaF2 ,
Write (i) ions occupying the voids (ii) types of voids occupied (iii) fraction of voids occupied.
(a)NaCl=Na+ ions in all the octahedral voids
(b)ZnS=Zn2+ ions in alternate tetrahedral voids
(c ) CaF2=F− ions in all the tetrahedral voids.
Topper's Solved these Questions
- STATES OF MATTER: SOLID MATTERBook:PRADEEPChapter:STATES OF MATTER: SOLID MATTERExercise:NCERT
Explore 49 Videos
- STATES OF MATTER: SOLID MATTERBook:PRADEEPChapter:STATES OF MATTER: SOLID MATTERExercise:NCERT (EXERCISE)
Explore 1 Video
- STATES OF MATTER: SOLID MATTERBook:PRADEEPChapter:STATES OF MATTER: SOLID MATTERExercise:TEST YOUR GRIP (FILL IN BLANKS)
Explore 46 Videos
- STATES OF MATTER : GASES AND LIQUIDESBook:PRADEEPChapter:STATES OF MATTER : GASES AND LIQUIDESExercise:VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)
Explore 12 Videos
- STRUCTURE OF ATOMBook:PRADEEPChapter:STRUCTURE OF ATOMExercise:Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))
Explore 12 Videos
Similar Questions
In each of the compounds : NaCl, ZnS and CaF2 ,
Write (i) ions occupying the voids (ii) types of voids occupied (iii) fraction of voids occupied.
View SolutionIn chromium (III) chloride CeCl3 chloride ions have cubic close packed arrangement and Cr (III) ions present in the octahedral voids. What fraction of the octahedral void is occupied ? What fraction of the total number of voids is occupied?
View Solution
Knowledge Check
Fraction of the total octahedral voids occupied will be :A12B14C18D16In Zinc blende , the percentage of tetrahedral voids occupied by Zn2+ ions is :
A25%B50%C75%D100%A solid is formed and it has three types of atoms X, Y and Z, X forms a fcc lattice with Y atoms occupying all tetrahedral voids and Z atoms occupying half of octahedral voids. The formula of solid is :-
AX4YZ2BX4Y2ZCXY2Z4DX2Y4Z
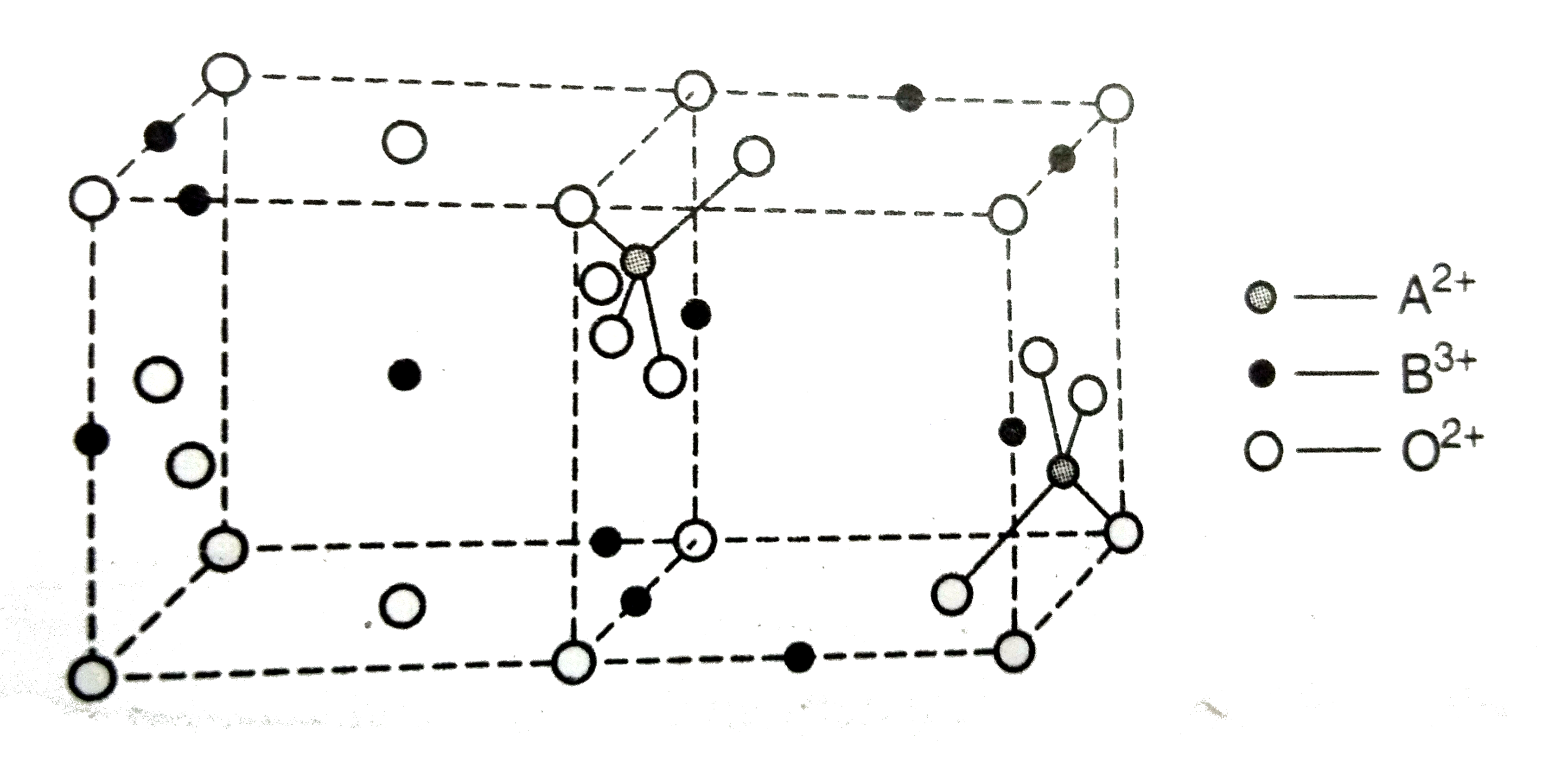
In the mineral, spinel, having the formula MgAl2O4 oxide ions ar arranged , in the cubic close packing, Mg2+ ions occupy the tetrahedrel voids while Al3+ ions occupy the octahedral voids.
(i) What precnetage of tetrahedral voids is occupied by Mg2+ ions ?
(ii) What precentage of octahedral voids is occupied by Al3+ ions ?
View SolutionIn a sodium chloride structure, the percentage of the octahedral voids occupied by cation is:
View SolutionIn the mineral, spinel, having the formula MgAl2O4 oxide ions ar arranged , in the cubic close packing, Mg2+ ions occupy the tetrahedrel voids while Al3+ ions occupy the octahedral voids.
(i) What precnetage of tetrahedral voids is occupied by Mg2+ ions ?
(ii) What precentage of octahedral voids is occupied by Al3+ ions ?
View SolutionNumber of tetrahedral voids occupied in a unit cell of diamond is
View SolutionA solid is formed and it has three types of atoms X, Y and Z, X forms a fcc lattice with Y atoms occupying all tetrahedral voids and Z atoms occupying half of octahedral voids. The formula of solid is :-
View Solution
