in which of the following structures coordination number for cations and anions in the packed structure will be same ?
Cl− ion from fcc lattice and Na+ ions occupy all octahedral voids of the unit cell
Ca2+ ions from fcc lattice and F− ions occupy all the eight tetrahedral voids of the unit cell
O2− ions from fcc lattice and Na+ ions occupy all the eight tetrahedral voids of the unit cell
S2− ions from fcc lattice and Zn2+ ions go into alternate tetrahedral voids of the unit cell
The correct Answer is:a
In Na+Cl−, all cations and anions have a coordination number of 6
टॉपर्स ने हल किए ये सवाल
- STATES OF MATTER: SOLID MATTERBOOK - PRADEEPCHAPTER - STATES OF MATTER: SOLID MATTEREXERCISE - NCERT EXEMPLAR PROBLEMS (MULTIPLE CHOICE- II)
16 Videos
- STATES OF MATTER: SOLID MATTERBOOK - PRADEEPCHAPTER - STATES OF MATTER: SOLID MATTEREXERCISE - NCERT EXEMPLAR PROBLEMS (SHORT ANSWER)
10 Videos
- STATES OF MATTER: SOLID MATTERBOOK - PRADEEPCHAPTER - STATES OF MATTER: SOLID MATTEREXERCISE - NCERT (EXERCISE)
1 Video
- STATES OF MATTER : GASES AND LIQUIDESBOOK - PRADEEPCHAPTER - STATES OF MATTER : GASES AND LIQUIDESEXERCISE - VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)
12 Videos
- STRUCTURE OF ATOMBOOK - PRADEEPCHAPTER - STRUCTURE OF ATOMEXERCISE - Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))
12 Videos
समान प्रश्न
ज्ञान जांच
in which of the following strutures corrding number for cations and anions in the packed structure will be same ?
ACl− ions form fcc lattice and Na+ ions occupy all octahedral voids of the unit cellBCa2+ ions form fcc lattice and F− ions occupy all the eight tetrahedral voids of the unit cellCO2− ions form fcc lattice and Na+ ions occupy all the eight tetrahedral voids of the unit cellDS2− ions form fcc lattice and Zn2+ ions go into go into alternate tetrahedral voids of the unit cellIn which of the following structures, the coordination number of both the ions are same ?
ACesium chlorideBSodium chlorideCZinc sulphideDSodium oxideIn the fluorite structure, the coordination number of cation is:
A4B6C8D3
For a solid with the following structure, the coordination number of the point B is
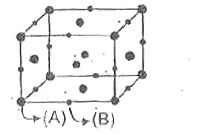
View SolutionFor a solid with the following structure, the coordination number of the point B is
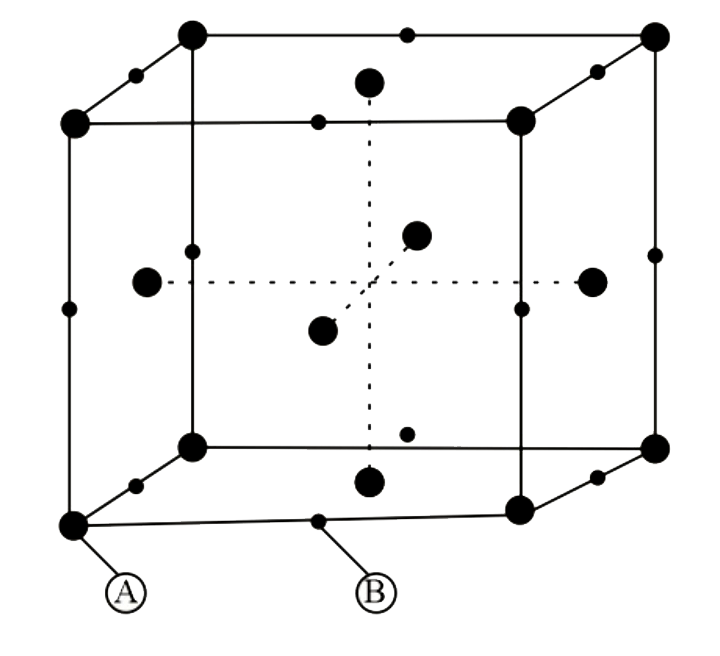
View SolutionIn which of the following structures, the anion has maximum coordination number?
View SolutionFor a solid with the following structure, the coordination number of the point B is :
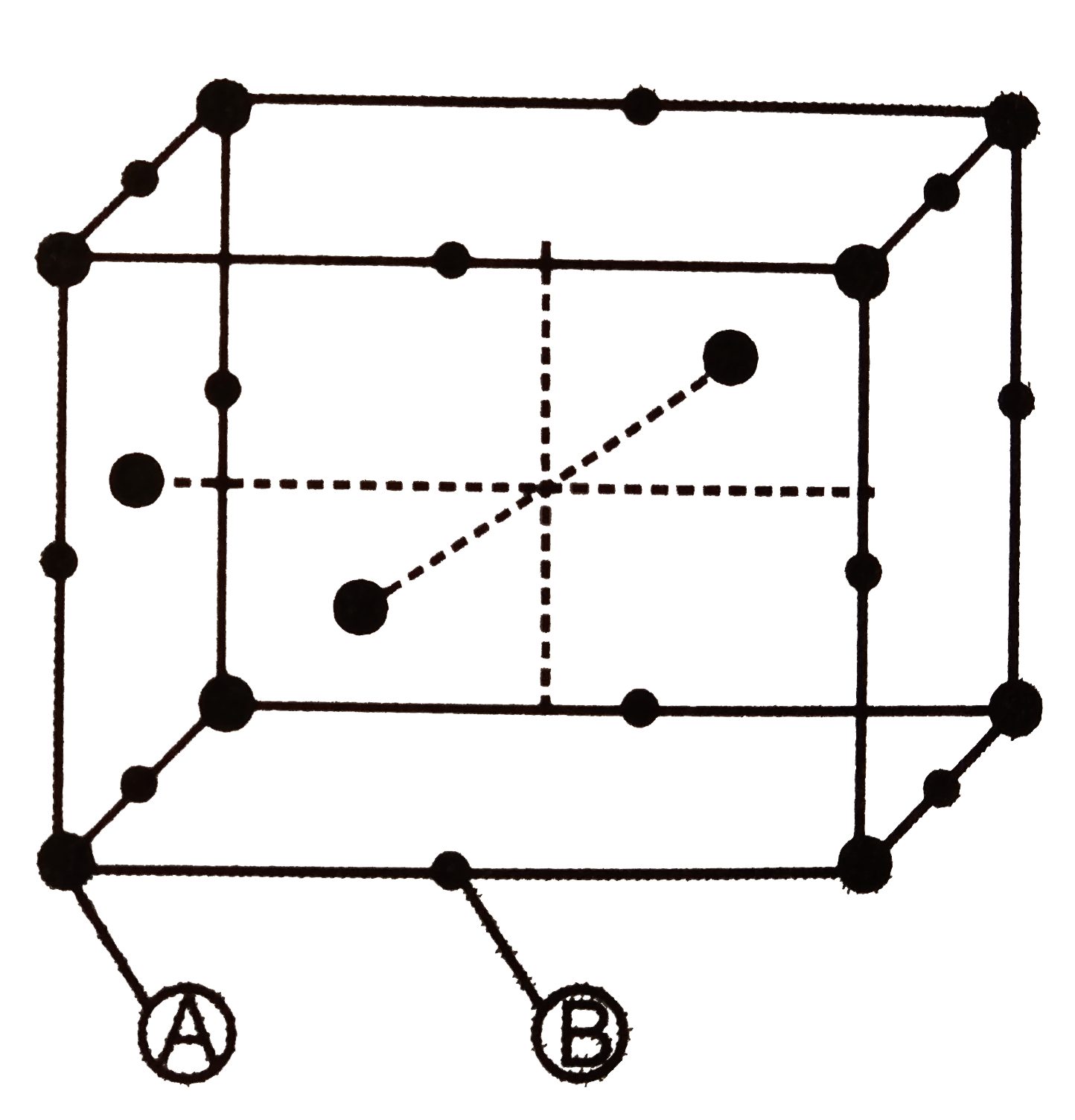
View SolutionIn the calcum fluaride structure, the coordination bumber of the cations and anions are respectively ,
View Solution

