(i) (a) Following is the schematic alignment of magnetic moments :
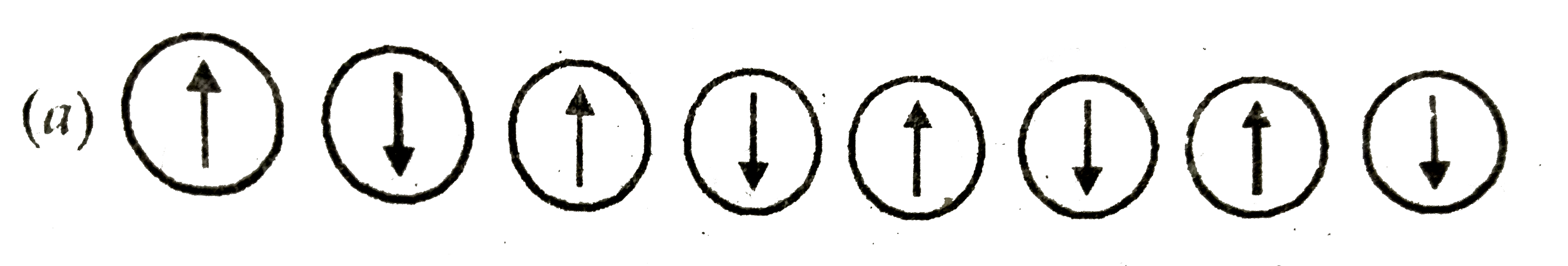
What type of magnetism is shown by this substance ?
(b) What type of stoichiometric defect is shown by (i) KCI (ii) AgCl ?
(ii) An element with density 11.2gcm−3 forms a fcc lattice with length of 4×d10−8 cm. Calculate the atomic mass of the element. (NA=6.02×1023mol−1).
(i)(a)Anti-ferromagnetic (b)(i)Schottky defect (ii)Frenkel defect , (iii)M=ρ×a3×NAZ=(11.2)(4×10−8)3(6.02×1023)4=107.9g mol−1
Topper's Solved these Questions
- STATES OF MATTER: SOLID MATTERBook:PRADEEPChapter:STATES OF MATTER: SOLID MATTERExercise:ANALYTICAL (QUESTION)
Explore 15 Videos
- STATES OF MATTER: SOLID MATTERBook:PRADEEPChapter:STATES OF MATTER: SOLID MATTERExercise:ANALYTICAL (PROBLEMS )
Explore 10 Videos
- STATES OF MATTER: SOLID MATTERBook:PRADEEPChapter:STATES OF MATTER: SOLID MATTERExercise:ADDITIONAL QUESTIONS ( SHORT ANSWER )
Explore 44 Videos
- STATES OF MATTER : GASES AND LIQUIDESBook:PRADEEPChapter:STATES OF MATTER : GASES AND LIQUIDESExercise:VIII. ASSERTION-REASON TYPE QUESTIONS (TYPE-II)
Explore 12 Videos
- STRUCTURE OF ATOMBook:PRADEEPChapter:STRUCTURE OF ATOMExercise:Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))
Explore 12 Videos
Similar Questions
Why ureas has a sharp melting point but glass does not ?
View SolutionLithium borohydride (LiBH)4 crystallizes in an orthorohombic system having 4 molecules per unit cell. The unit cell dimensions are : a=6.81Åandc=7.17Å. Calculate the density of the crystal (At. Mass of Li=7,B=11,H=1u).
View Solution(a)Write two difference between crystalline solids and amorphous solids ?
(b)Draw a diagram for anisotropic behaviour of crystalline solids .
View SolutionAssertion. London forces of are the attractive forces existing among non-polar molecules or noble gases.
Reason. London forces are the attractive forces which operate at all distances between the molecules atoms.
View SolutionAssertion. Cathode rays originate from the cathode and move towards andoe.
Reason. Electrons present in the material of the cathode are continuously emitted on applying high voltage
View SolutionAn element with density 11.2g cm−3 forms a fcc lattice with edge length of 4×10−8 cm. Calculate the atomic mass of the element.
View Solution(i) (a) Following is the schematic alignment of magnetic moments :

What type of magnetism is shown by this substance ?
(b) What type of stoichiometric defect is shown by (i) KCI (ii) AgCl ?
(ii) An element with density 11.2gcm−3 forms a fcc lattice with length of 4×d10−8 cm. Calculate the atomic mass of the element. (NA=6.02×1023mol−1).
View SolutionSilver crystallises in a fcc lattice. The edge length of its unit is 4.077xx10^(-8)cm and its density is 10.5g cm^(-3) . Claculate on this basis of the atomic mass of silver (N_(A)=6.02xx10^(23) "mol"^(-1))
View SolutionAn element with density 11.2gcm−3 forms a f. c. c. lattice with edge length of 4×10−8 cm. Calculate the atomic mass of the element. (Given : NA=6.022×1023mol−1
View SolutionAn element with density 11.2 g cm^(-3) forms a f.c.c lattic with edge lengh of 4 xx 10^(-8) cm. calculate the of atoms mass of the element ( Given : N_(A) = 6.022 xx 10^(23) mol^(-1)
View SolutionAn element with density 11.2 g cm^(-3) forms a f.c.c. lattice with the edge length of 4xx10^(-8) cm. Calculate the atomic mass of the element. (Given : N_A=6.022xx10^23 "mol"^(-1) )
View SolutionWhat type of stoichiometric defect is shown by : (i) ZnS (ii)AgBr?
View SolutionAn element with density 2.8g*cm^(-3) forms a fcc unit cell with edge length 4xx10^(-8) cm. Calculate the molar mass of the element. [Given: N_(A)=6.022xx10^(23)mol^(-1)]
View Solution(a) An element crystallises in a cubic crystal structure with unit cell's edge length of 3.15Å. The atomic mass and the density of the element are 96 amu and 10.2g⋅cm−3. Predict the crystal lattice possessed by the element. (b) What type of stoichiometric defects is shown by AgCl?
View SolutionAn element with density 11.2gcm−3 forms a fcc lattice with edge length of 4×10−8 cm. Calculate the atomic mass of the element. [Given:NA=6.022×1023atoms mol−1]
View SolutionAn element with density 11.2gcm−3 forms a fcc lattice with edge length of 4×10−8 cm. Calculate the atomic mass of the element. Give NA=6.022×1023 atoms mol−1.
View Solution
