Briefly explain the following :
(i) Heat of combustion (ii) Bond energy .
Video Solution
|
Answer
Step by step video solution for Briefly explain the following : (i) Heat of combustion (ii) Bond energy . by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBOOK - PRADEEPCHAPTER - THERMODYNAMICSEXERCISE - ADDITIONAL QUESTIONS(VERY SHORT ANSWER QUESTIONS)
43 Videos
- THERMODYNAMICSBOOK - PRADEEPCHAPTER - THERMODYNAMICSEXERCISE - SHORT ANSWER QUESTIONS
65 Videos
- THERMODYNAMICSBOOK - PRADEEPCHAPTER - THERMODYNAMICSEXERCISE - ASSERTION AND REACTION TYPES QUESTIONS
3 Videos
- STRUCTURE OF ATOMBOOK - PRADEEPCHAPTER - STRUCTURE OF ATOMEXERCISE - Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))
12 Videos
Similar Questions
Knowledge Check
The heat of combustion is
AAlways -veBAlways +veCZeroDMay be +ve or -veThe enthalpy combustion of a substance
AIs always positiveBIs always negativeCIs equal to heat of formationDNothing can be said without reactionWhich of the following has highest heat of combustion ?
A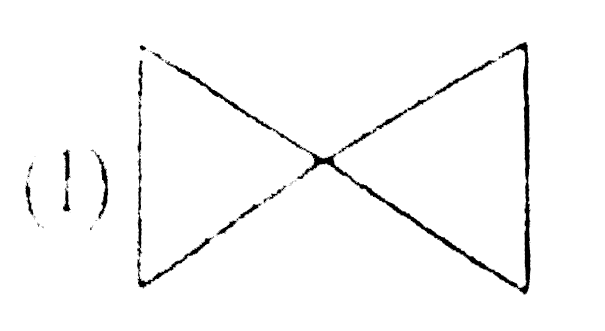 B
B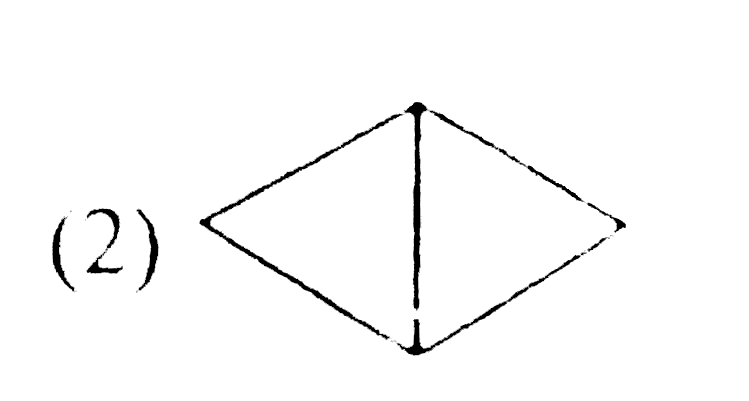 C
C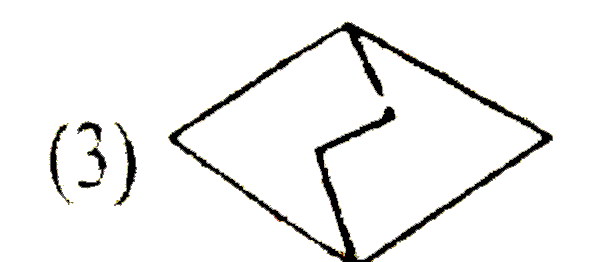 D
D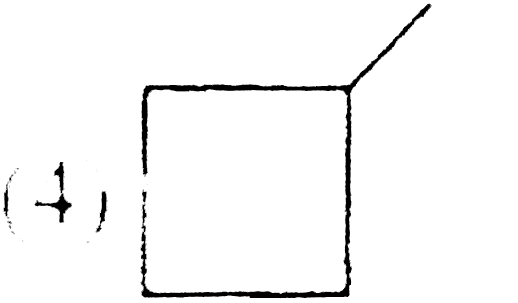
Briefly explain : Water gas
View Solution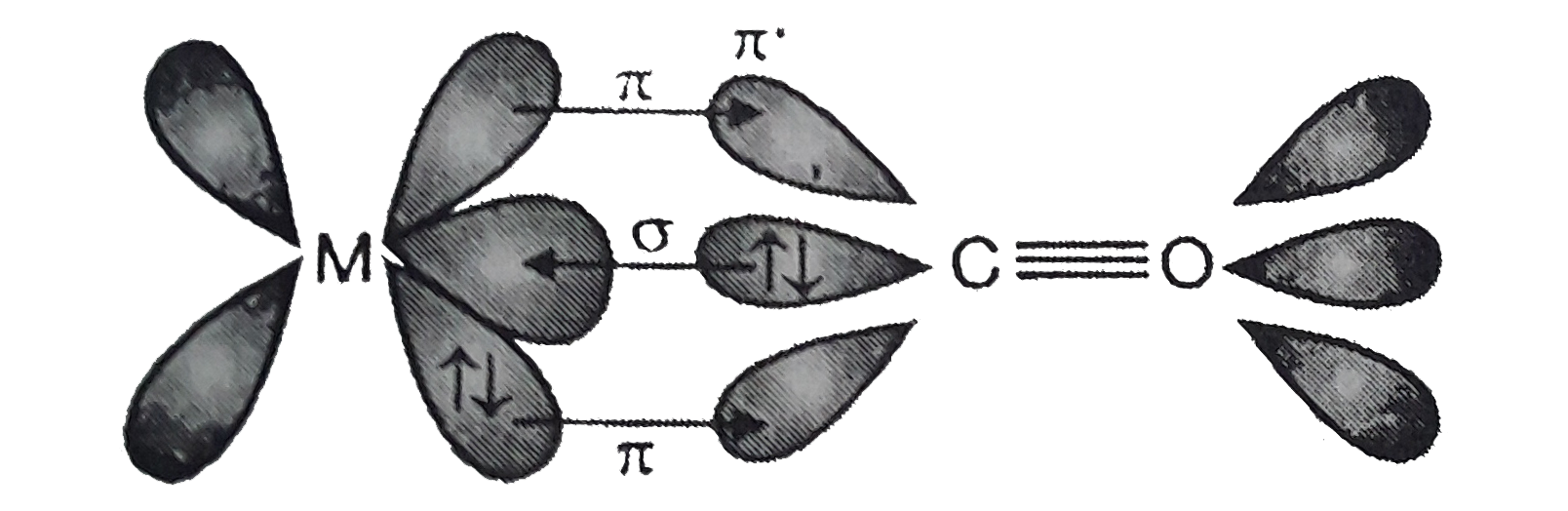
The figure represents the synergic bonding interaction in metal carbonyl complex. On the basis of the explain the following:
(i) Strength of Metal-ligand bond
(ii) Bond order of CO in carbonyl complex as compared to bond order in carbon monoxide.
View SolutionBriefly explain the term 'Goods'
View SolutionBriefly explain why the following reaction does not occur
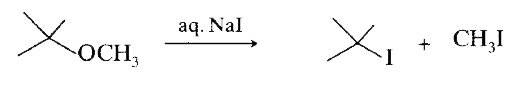
View SolutionAmong the following gases, heat of combustion is highest for
View Solution

