An ideal gas is expand from (p1,V1,T1) to (p2,V2,T2) under different conditions. The correct statement(s) among the following is (are):
The work done by the gas is less when it is expanded reversibly from V1 to V2under adiabatic conditions as compared to that when expanded reversibley from V1to V2 under isothermal conditions.
The change in internal energy of the gas is (i) zero, if it isexpanded reversibly with T1=T2, and (ii) positive, if it is expanded reversibly under adaibatic conditions with T_(1) cancel(=) T_(2)
If the expansion is carried out freely , it is simulataneously both isothermal as well as adiabatic
The work done on the gas is maximum irreveribly from (p2,V2) to (p1,V1) against constant pressurep1.
The correct Answer is:a,c,d
(a) 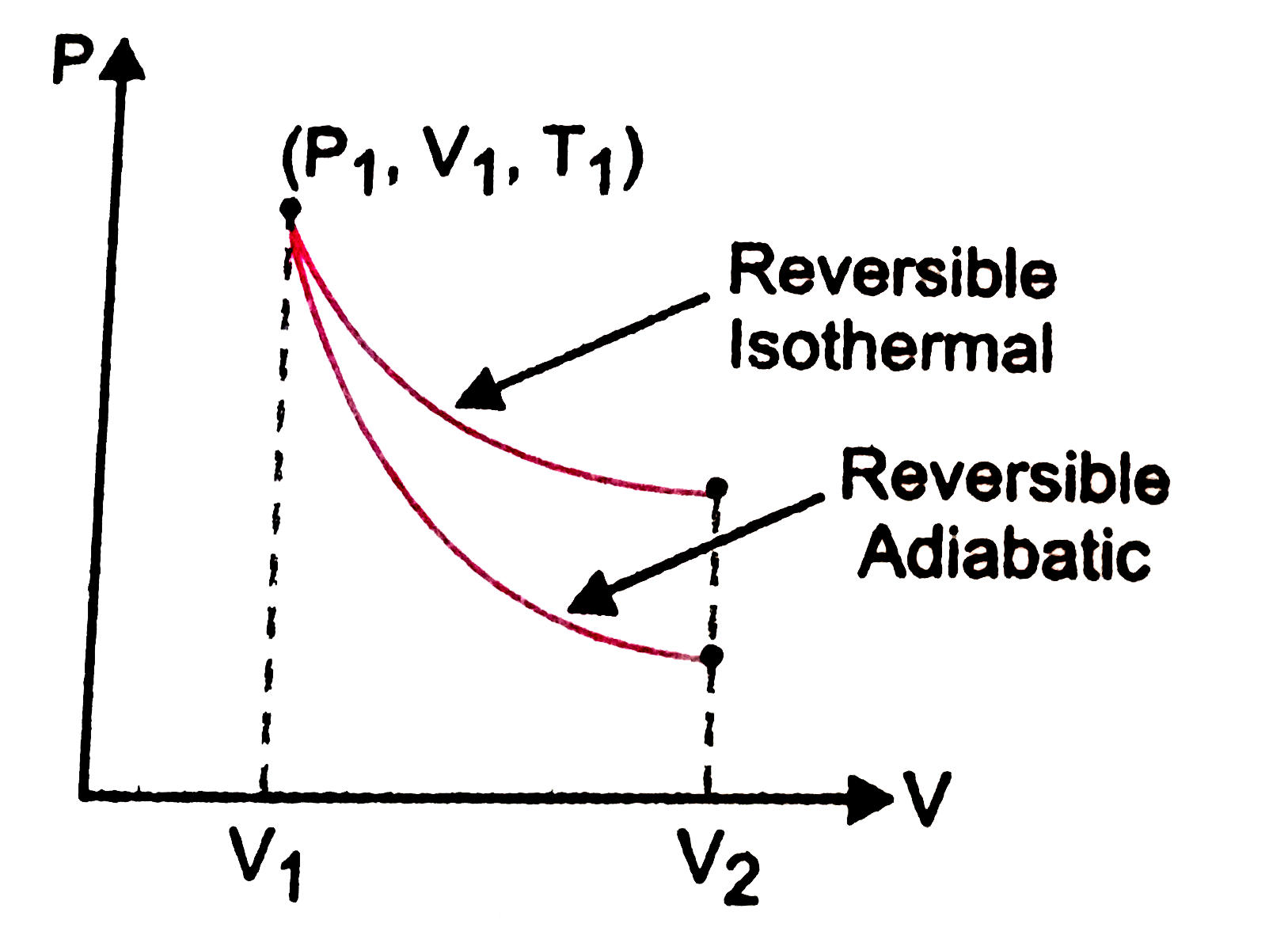
Area under the curve of reversible isothermal expansion is more than that under the adiabatic curve. Hence, work done in reversible isothermal expansion is more.
(b) For reversible expansion with T1=T2 means isothermal expansion.For reversible isothermal expansion of a gas, ΔU=0⟨'ΔU=nCvΔT) In reversible adiabatic expansion, T2<T91)
i.e., ΔT=−ve,∴ΔU=nCvΔT=−ve ( and not +ve)
(c ) In free expansion , Pext=0∴w=0
If process is carried out isothermally , ΔU=0. Hence, by 1st law , ΔU=q+w or q=ΔU−w=0−0=0 ( i.e. adiabatic).
If process is carried out adiabatically , q=0.
As w=0, therefore,by 1st law, ΔU=q+w=0 ( isothermal).
(d) 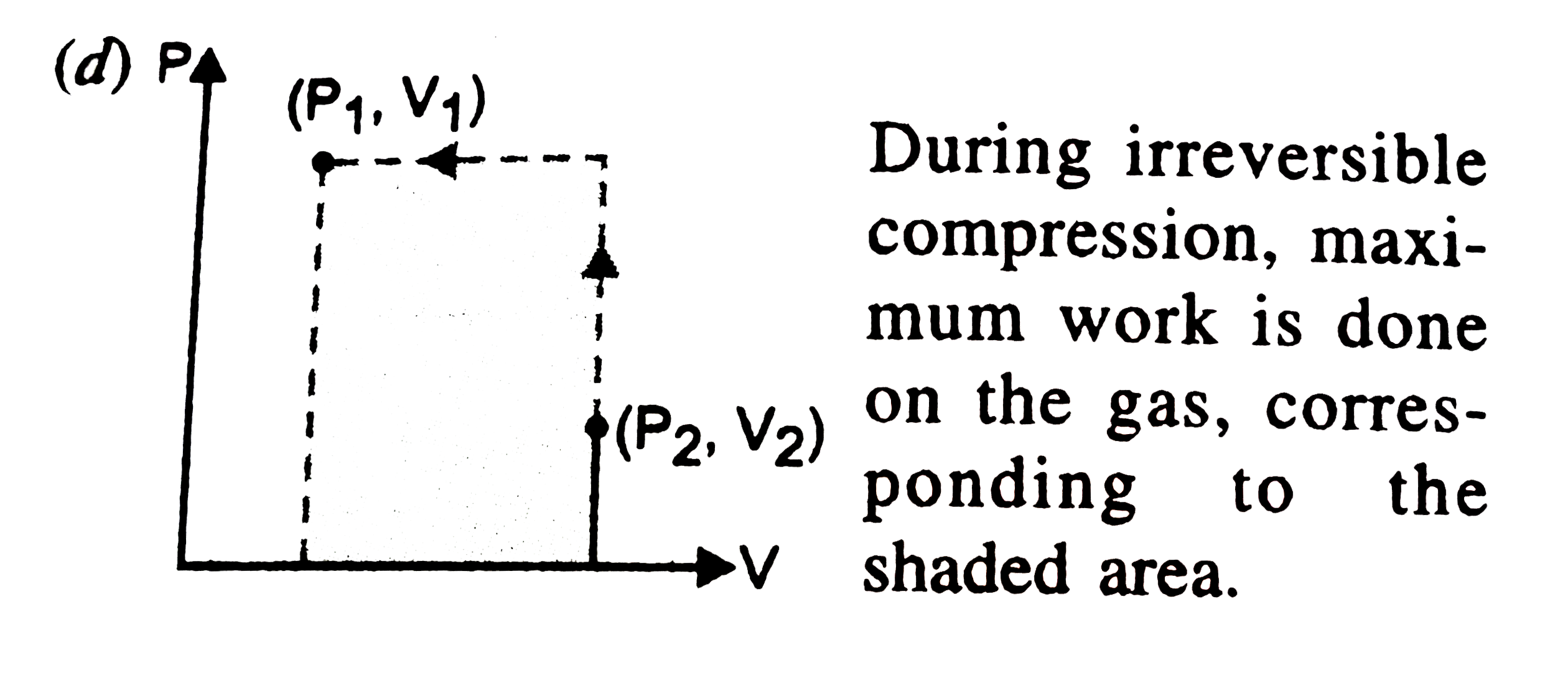 During irrevesible compression,maximum work is done on the gas, corresponding to the shaded area.
During irrevesible compression,maximum work is done on the gas, corresponding to the shaded area.
Topper's Solved these Questions
- THERMODYNAMICSBOOK - PRADEEPCHAPTER - THERMODYNAMICSEXERCISE - Comprehension 1
1 Video
- THERMODYNAMICSBOOK - PRADEEPCHAPTER - THERMODYNAMICSEXERCISE - Comprehension 2
1 Video
- THERMODYNAMICSBOOK - PRADEEPCHAPTER - THERMODYNAMICSEXERCISE - SHORT ANSWER QUESTIONS
65 Videos
- STRUCTURE OF ATOMBOOK - PRADEEPCHAPTER - STRUCTURE OF ATOMEXERCISE - Competition Focus (JEE (Main and Advanced)/Medical Entrance (IX. Assertion And Reason Type Questions (Type II))
12 Videos
Similar Questions
In a fuel celll, methanol is used as a fuel and O2 is used as oxidizer. The standard enthalpy of combustion of methanol is -726 kJ mol−1. The standard free energies of formation of CH3OH(I),CO2(g)andH2O(I) are -166.3, -394.4 and -237.1 kJ mol−1 respectively.
The standard free energy change of the reaction will be
View SolutionIn a fuel cell (device used for producing electricity directly from a chemical reaction )methanol is used as a fuel and oxygen gas is used asan oxidizer. The standard enthalpy of combustion of methanol is −721kJmol−1 . The standard free energiesof formation of CH3OH(l,CO2(g) and H2O(l) are- 166.3 , -394.4 and −237.1kJmol−1 respectively.
The efficiency of the fuel cell will be
View SolutionWhat do you understand by open, closed and isolated system ? Illustrate with suitable examples.
View SolutionAssertion. Cathode rays originate from the cathode and move towards andoe.
Reason. Electrons present in the material of the cathode are continuously emitted on applying high voltage
View SolutionFor an ideal gas, an illustratio of three different paths A(B+C) and (D+E) from an initial state P1,V1,T1 to a final state P2,V2,T1 is shown in the given figure.
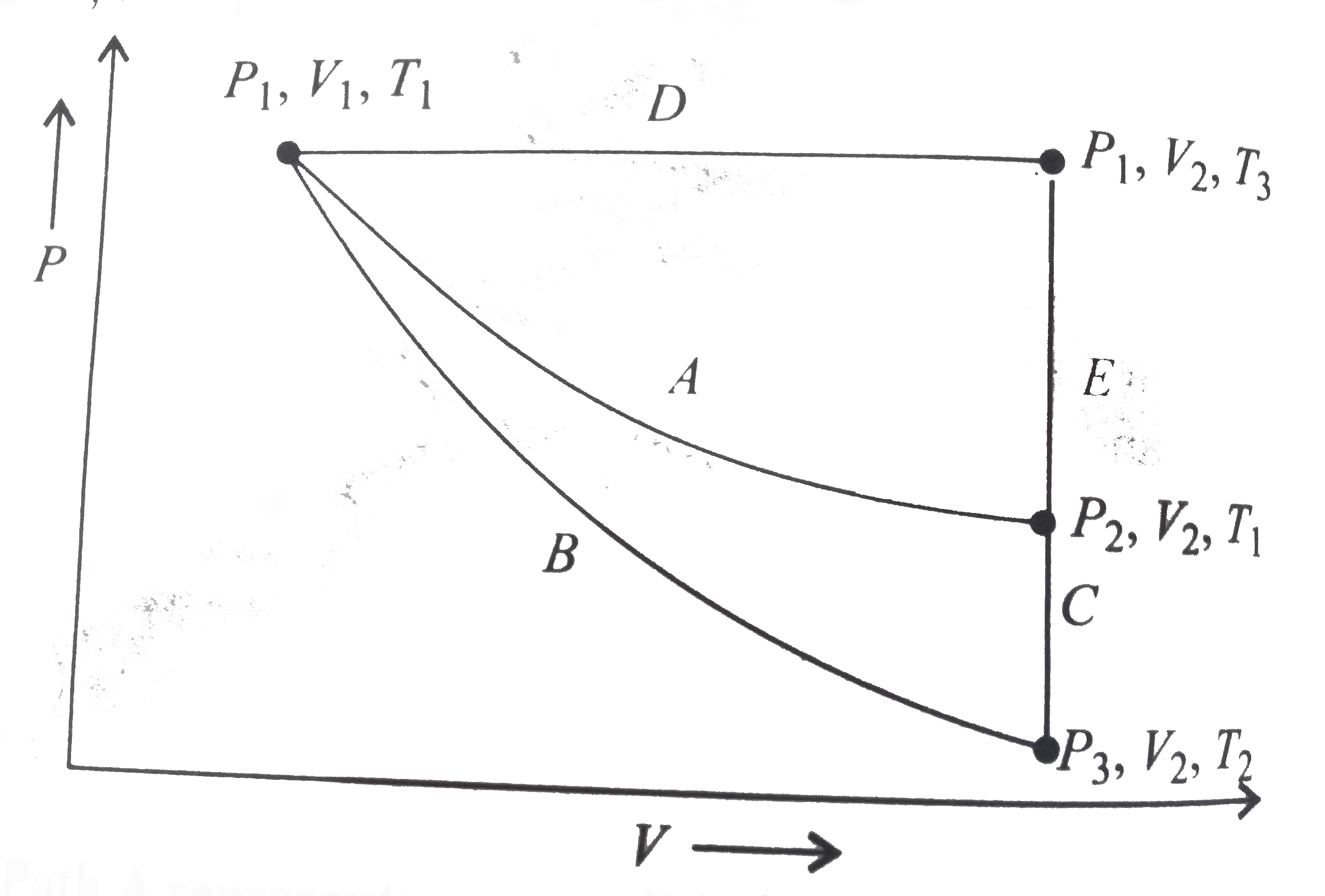
Path Arepresents a reversible isothermal expansion form P1,V1 to P2,V2, Path (B+C) represents a reversible adiabatic expansion (B) from P1,V1,T1→P3,V2,T2 followed by reversible heating the gas at constant volume (C)from P3,V2,T2 to P2,V2,T1. Path (D+E) represents a reversible expansion at constant pressure P1(D) from P1,V1,T1 to P1,V2,T3 followed by a reversible cooling at constant volume V2(E) from P1,V2,T3→P2,V2,T1.
What is qrev, for path (A)?
View SolutionFor an ideal gas, an illustratio of three different paths A(B+C) and (D+E) from an initial state P_(1), V_(1), T_(1) to a final state P_(2), V_(2),T_(1) is shown in the given figure. Path A represents a reversible isothermal expansion form P_(1),V_(1) to P_(2),V_(2) , Path (B+C) represents a reversible adiabatic expansion (B) from P_(1),V_(1),T_(1)to P_(3),V_(2),T_(2) followed by reversible heating the gas at constant volume (C) from P_(3),V_(2),T_(2) to P_(2),V_(2),T_(1) . Path (D+E) represents a reversible expansion at constant pressure P_(1)(D) from P_(1),V_(1),T_(1) to P_(1),V_(2),T_(3) followed by a reversible cooling at constant volume V_(2)(E) from P_(1),V_(2),T_(3) to P_(2),V_(2),T_(1) . What is q_(rev) , for path (B +C) ?
View SolutionFor an ideal gas, an illustratio of three different paths A(B+C) and (D+E) from an initial state P1,V1,T1 to a final state P2,V2,T1 is shown in the given figure.
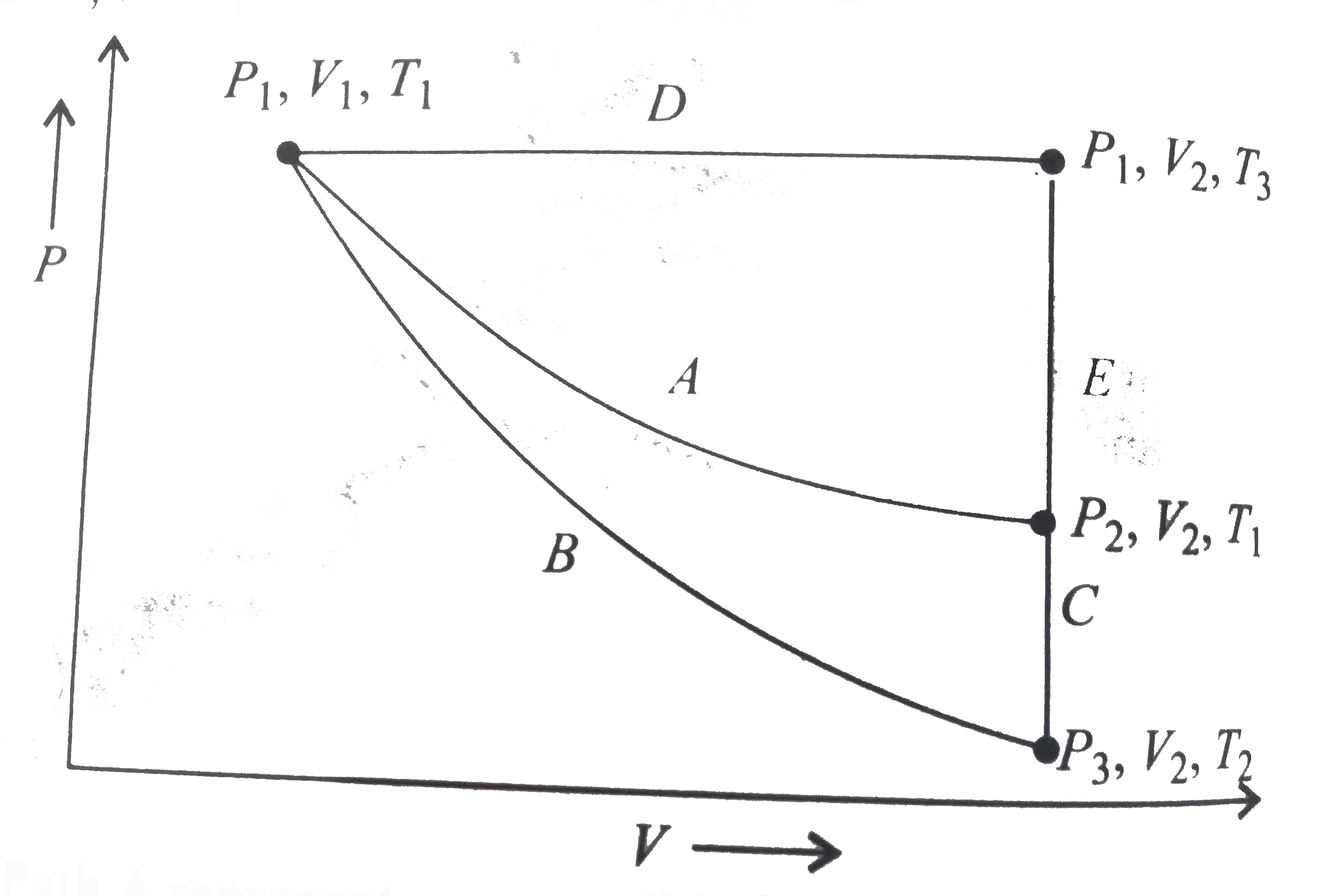
Path Arepresents a reversible isothermal expansion form P1,V1 to P2,V2, Path (B+C) represents a reversible adiabatic expansion (B) from P1,V1,T1→P3,V2,T2 followed by reversible heating the gas at constant volume (C)from P3,V2,T2 to P2,V2,T1. Path (D+E) represents a reversible expansion at constant pressure P1(D) from P1,V1,T1 to P1,V2,T3 followed by a reversible cooling at constant volume V2(E) from P1,V2,T3→P2,V2,T1.
What is ΔS for path A?
View SolutionFor an ideal gas, an illustratio of three different paths A(B+C) and (D+E) from an initial state P1,V1,T1 to a final state P2,V2,T1 is shown in the given figure.
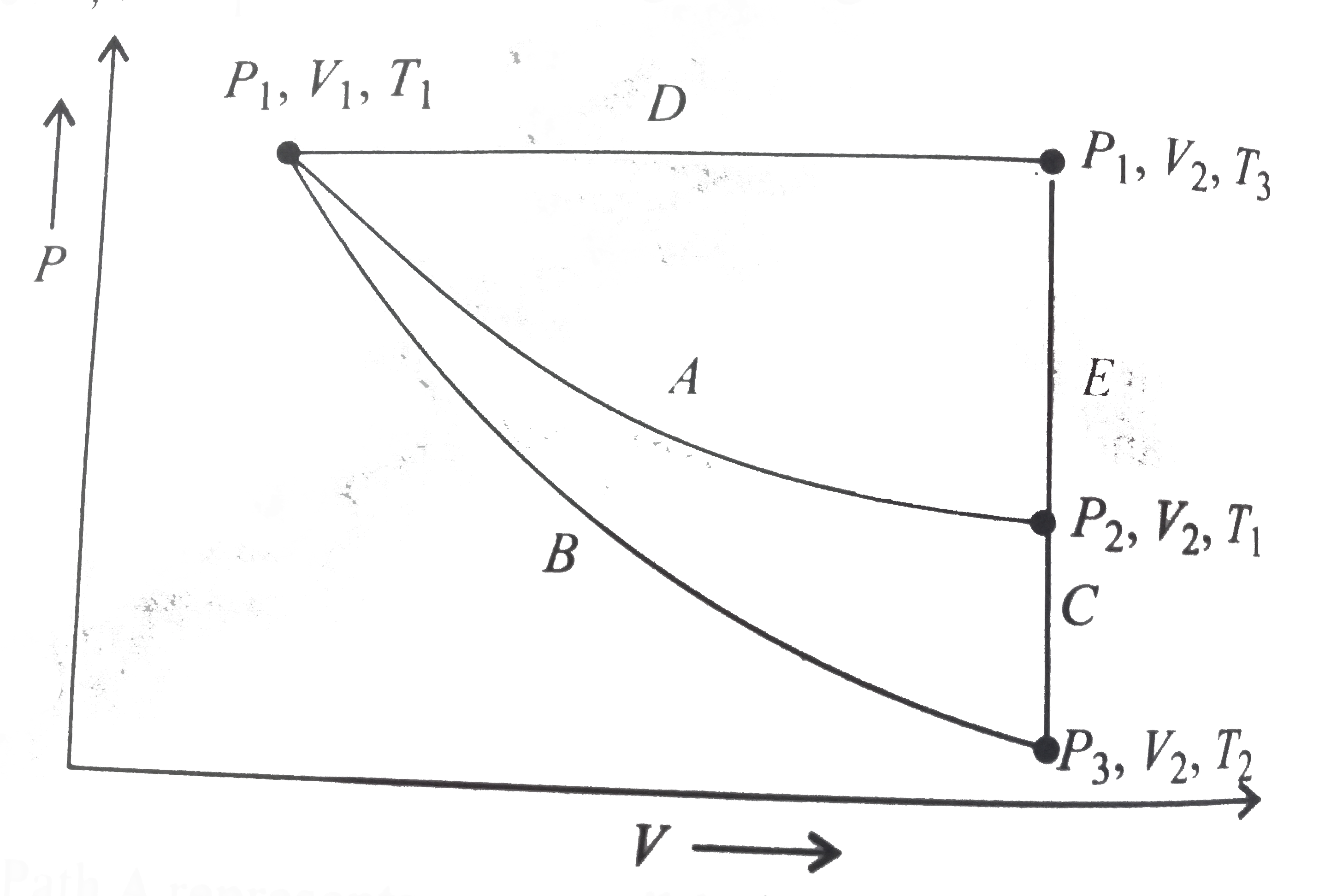
Path Arepresents a reversible isothermal expansion form P1,V1 to P2,V2, Path (B+C) represents a reversible adiabatic expansion (B) from P1,V1,T1→P3,V2,T2 followed by reversible heating the gas at constant volume (C)from P3,V2,T2 to P2,V2,T1. Path (D+E) represents a reversible expansion at constant pressure P1(D) from P1,V1,T1 to P1,V2,T3 followed by a reversible cooling at constant volume V2(E) from P1,V2,T3→P2,V2,T1.
What is qrev, for path (D+E)?
View SolutionAn ideal gas follows a process described by PV2=C from (P1,V1,T1) to (P2,V2,T2) (C is a constant). Then
View SolutionAn ideal gasa undergoes through folowing cyclic procees. 1-2 : Reversible adialbatic comparession from P_(1)V_(1)T_(1)"to"P_(2)V_(2)T_(2) 2-3 : Reversible isochoric heating from P_(2)V_(2)T_(2)" to " P_(3)V_(3)T_(3) 3-4 Reversible adialbatic expansion from P_(3)V_(3)T_(3)" to " P_(4)V_(4)T_(4) 4-1 Reversible isochoric cooling from P_(4)V_(4)T_(4)" to " P_(1)V_(1)T_(1) Eefficiencyof the cycle is :
View SolutionFor an ideal monoatomic gas, an illustration of three different paths A,(B+C) and (D+E) from an initial state P_(1),V_(1),T_(1) to a final state P_(2),V_(2),T_(1) is shown in the given figure . Path A represents a reversible isothermal from P_(1)V_(1)" to "P_(2),V_(2) , path (B+C) represent a reversible adiabatic expansion (B) from P_(1),V_(1),T_(1)" to " P_(3),V_(2),T_(2) followed by reversible heating of the gas at constant volume (C) from P_(3),V_(2),T_(2) " to " P_(2),V_(2),T_(1) . Path (D+E) represents a reversible expansion at constant pressure P_(1)(D) from P_(1),V_(1),T_(1) " to "P_(1),V_(2),T_(3) followed by a reversible cooling at constant volume V_(2)(E) form P_(1),V_(2),T_(3) " to "P_(2),V_(2),T_(1) What is q_("rev") for path (A)?
View SolutionFor an ideal monoatomic gas, an illustration of three different paths A,(B+C) and (D+E) from an initial state P1,V1,T1 to a final state P2,V2,T1 is shown in the given figure .
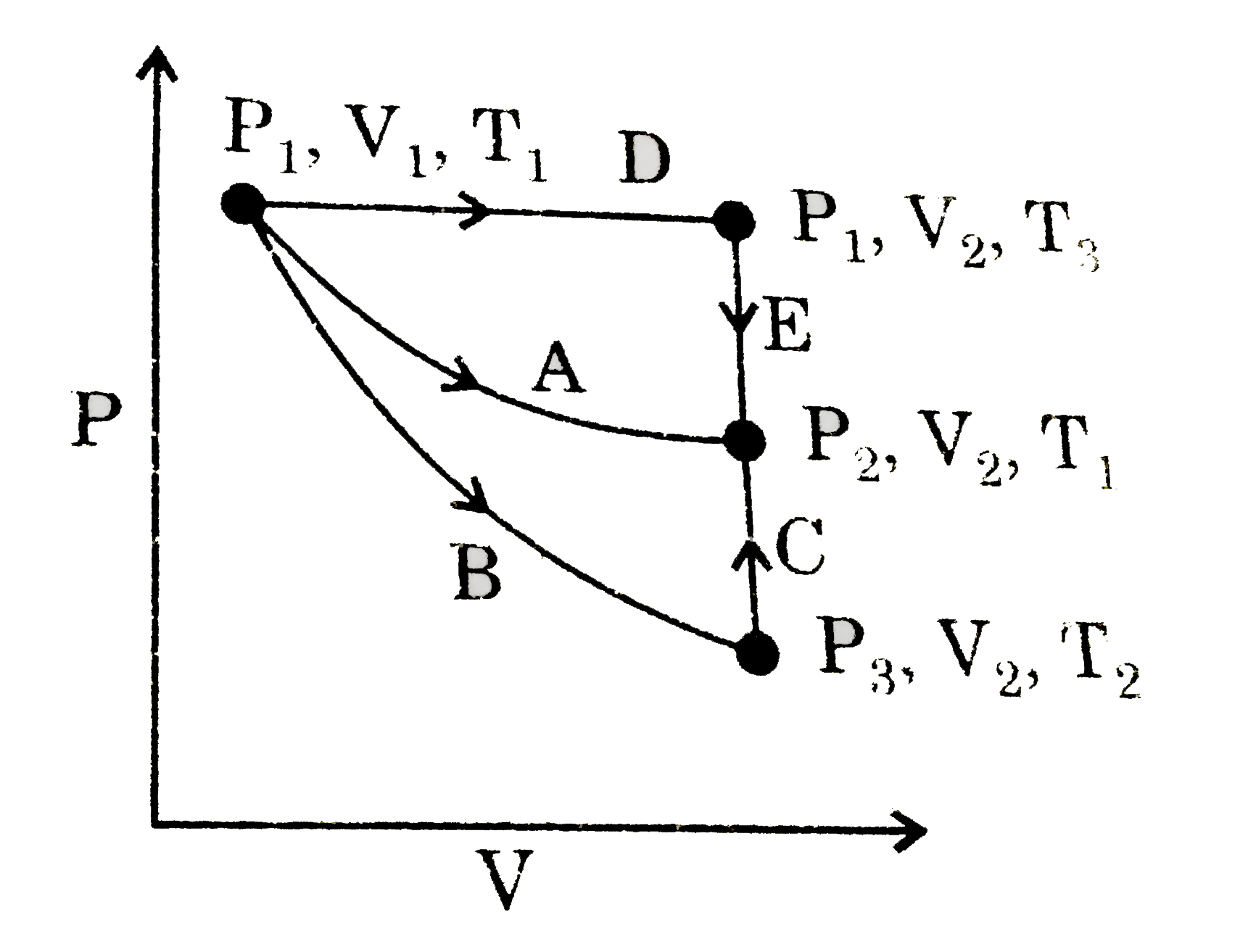
Path A represents a reversible isothermal from P1V1 to P2,V2, path (B+C) represent a reversible adiabatic expansion (B) from P1,V1,T1 to P3,V2,T2 followed by reversible heating of the gas at constant volume (C) from P3,V2,T2 to P2,V2,T1. Path (D+E) represents a reversible expansion at constant pressure P1(D) from P1,V1,T1 to P1,V2,T3 followed by a reversible cooling at constant volume V2(E) form P1,V2,T3 to P2,V2,T1
What is ΔS for (D+E)?
View SolutionAn ideal gas is expanded from (p1,V1,T1) to (p2,V2,T2) under different conditions. The correct statement(s) among the following is (are).
View SolutionAn ideal gas is expanded from (P_(1), V_(1), T_(1)) to (P_(2), V_(2), T_(2)) under different conditions. The correct statement(s) among the following is (are)
View SolutionAn ideal gas is expand from (p1,V1,T1) to (p2,V2,T2) under different conditions. The correct statement(s) among the following is (are):
View Solution

