(i) Draw the gas phase and solid phase structure of H2O2.
(ii) H2O2 is a better oxidising agnet than water . Explain .
Video Solution
Text Solution
(ii) H2O2 is a better oxidising agent than water as discussed below:
(a) H2O2 oxidises an acidified solution of KI to give I2 which gives blue colour with starch solution but H2O does not
(b) H2O2 turns black PbS to white PbSO4 but H2O does not
|
Topper's Solved these Questions
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:NCERT EXEMPLAR PROBLEMS WITH ANSWER, HINTS AND SOLUTIONS (Assertion and Reason Type Questions)
Explore 2 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:NCERT EXEMPLAR PROBLEMS WITH ANSWER, HINTS AND SOLUTIONS (LONG ANSWER QUESTIONS)
Explore 8 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:NCERT EXEMPLAR PROBLEMS WITH ANSWER, HINTS AND SOLUTIONS (NCERT Exemplar Problems Chapter 9 Hydrogen) Multiple Choice Questions-II
Explore 9 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS
Explore 31 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:Competition Focus (Jee (Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions
Explore 25 Videos
Similar Questions
Knowledge Check
The structure of H2O2 is
A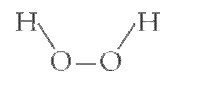 B
B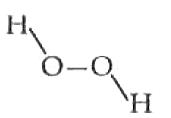 CH−O−O−HD
CH−O−O−HD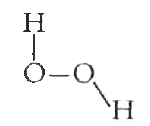
The structure of H2O2 is
AOpen book likeBLinearCClosed bookDPyramidal.The structure of H2O2 is
ALinearBplanarCthree dimensionalDboat type
