For each of the following compounds, write a comdensed formula and also their bond-line formula.
(a)HOCH2CH2CH2CH(CH3)CH(CH3)CH3 , (b)N≡C−OH∣CH−C≡N
Condensed Formulae (a) HO(CH2)3CH(CH3)CH(CH3)2 (b) HOCH(CN)2
Bond-line Formulae 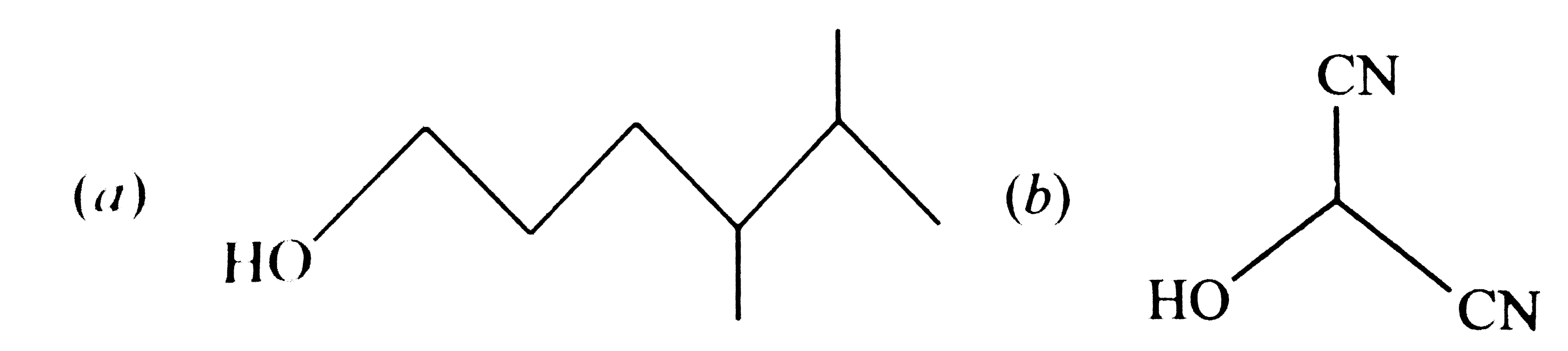
Topper's Solved these Questions
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:Curiosity Questions
Explore 7 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:Problem For Practice
Explore 22 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
- P-BLOCK ELEMENTS (NITROGEN FAMILY)Book:PRADEEPChapter:P-BLOCK ELEMENTS (NITROGEN FAMILY)Exercise:Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)
Explore 10 Videos
Similar Questions
Why is methane tetrahedral ?
View SolutionWhat is wrong with the following names ? Draw the structures they represent and give their correct name
(i) 1, 1-Dimethylpentane (ii) 2-Methyl-2-propylhexane (iii) 3,4-Dimethylpentane
(iv) 4, 4-Dimethyl-3-ethylpentane (v) 4-(2-Methylethyl)heptane
View SolutionAssertion : The water gas shift reaction can be used to increase the amount of H2 in the shift ' syn gas ' mixture.
Reason : In this reaction, water is reduced to H2 by CO.
View SolutionStatement -1 : Although NaH2PO2 contains two H-atoms, it is not an acid salt.
Satement -2 : It contains two ionisable hydrogens.
View SolutionWhat is the maximum number of steroisomers possible for each compound?
(a) CH3CH(OH)CH(OH)CH2CH3 (b) CH3CH2CH2CH(CH3)2
View SolutionFor each of the following compounds, write a condensed formula and also their bond-line formula.
(a)HOCH2CH2CH2CH(CH3)CH(CH3)CH3
(b) N≡C−OH∣CH−C≡N
View SolutionExpand each the following condensed formulae into their complete structural and bond line formulae:
(a)CH3CH2COCH2CH3 ,(b)CH3CH=CH(CH2)3CH3
View SolutionFor each of the following compounds, write a comdensed formula and also their bond-line formula. (a) HOCH_(2)CH_(2)CH_(2)CH(CH_(3))CH(CH_(3))CH_(3) , (b) N-=C-oversetoverset(OH)(|)(CH)-C-=N
View SolutionWrite its bond line formula
(i) CH3CH(OH)CH2CH2OH
View Solutionनिम्नलिखित यौगिकों के IUPAC नाम लिखिए-
(i) CH3 |CH3−CH2−CH−CH−C−CH3 | | | CH3 I CH3
(ii) Cl |CH3−CH2−CH−CH−C−CH3 | | | CH3 OH CH3
View SolutionFor each of the following compounds, write a condensed formula and also their bond-line formula. (a) HOCH_(2)CH_(2)CH_(2)CH(CH_(3))CH(CH_(3))CH_(3) (b) N-=C-overset(OH)overset("| ")(CH)-C-=N
View SolutionThe IUPAC name of the compound CH_(3) - oversetoverset(OH)(|)CH -CH_(2) - oversetoverset(CH_(3))(|)CH -CHO is
View SolutionFor each of the following compounds, write a condensed formula and also their bond-line formula.
(a)HOCH2CH2CH2CH(CH3)CH(CH3)CH3
(b) N≡C−OH∣CH−C≡N
View SolutionFor the following compounds, write their bondline formula.
(a) CH3CH(CH3)CH(CH3)CH2CH2OH (b) N≡C−CH∣OH−C≡N
View SolutionExpand each of the following condensed formulas into their completes structural formulas:
(a) CH3CH2CH2COCH2CH3
(b) CH3CH=CH(CH2)2CH3
View Solution
